Novel parvovirus and related variant in human plasma
- PMID: 16494735
- PMCID: PMC3291395
- DOI: 10.3201/eid1201.050916
Novel parvovirus and related variant in human plasma
Abstract
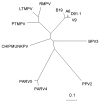
We report a novel parvovirus (PARV4) and related variants in pooled human plasma used in the manufacture of plasma-derived medical products. Viral DNA was detected by using highly selective polymerase chain reaction assays; 5% of pools tested positive, and amounts of DNA ranged from <500 copies/mL to >106 copies/mL plasma.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
