Age-related effects on nelfinavir and M8 pharmacokinetics: a population study with 182 children
- PMID: 16495250
- PMCID: PMC1426418
- DOI: 10.1128/AAC.50.3.910-916.2006
Age-related effects on nelfinavir and M8 pharmacokinetics: a population study with 182 children
Abstract
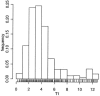
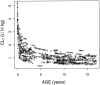
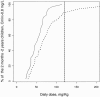
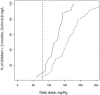
As a relationship between nelfinavir antiretroviral efficacy and plasma concentrations has been previously established, nelfinavir pharmacokinetics was investigated in order to optimize the individual treatment schedule in a pediatric population. A population pharmacokinetic model was developed to describe the concentration-time course of nelfinavir and its active metabolite M8. Individual characteristics were used to explain the large interindividual variability in children. Data from therapeutic drug monitoring in 182 children treated with nelfinavir were analyzed with NONMEM. Then Food and Drug Administration (FDA) current recommendations were evaluated estimating the percentage of children who reached the target minimum plasma concentration (0.8 mg/liter) by using Bayesian estimates. Nelfinavir pharmacokinetics was described by a one- compartment model with linear absorption and elimination. Pharmacokinetic estimates and the corresponding intersubject variabilities for the model were as follows: nelfinavir total clearance, 0.93 liters/h/kg (39%); volume of distribution, 6.9 liters/kg (109%); absorption rate, 0.5 h(-1); formation clearance fraction to hydroxy-tert-butylamide (M8), 0.025; M8 elimination rate, 1.88 h(-1) (49%). Apparent nelfinavir total clearance and volume of distribution decreased as a function of age. M8 elimination rate was increased by concomitant administration of nevirapine or efavirenz. Our data confirm that the FDA recommendations for children from 2 to 13 years are optimal and that the dose recommended for children younger than 2 years is adequate for the children from 2 months to 2 years old. However, in children younger than 2 months, the proposed nelfinavir newborn dose of 40 mg/kg of body weight twice daily is inadequate and we suggest increasing the dose to 50 to 60 mg/kg administered thrice daily. This assumption should be further evaluated.
Figures



References
-
- Anderson, B. J., R. A. van Lingen, T. G. Hansen, Y. C. Lin, and N. H. Holford. 2002. Acetaminophen developmental pharmacokinetics in premature neonates and infants: a pooled population analysis. Anesthesiology 96:1336-1345. - PubMed
-
- Baede-van Dijk, P. A., P. W. Hugen, C. P. Verweij-van Wissen, P. P. Koopmans, D. M. Burger, and Y. A. Hekster. 2001. Analysis of variation in plasma concentrations of nelfinavir and its active metabolite M8 in HIV-positive patients. AIDS 15:991-998. - PubMed
-
- Beal, S. L. 2001. Ways to fit a PK model with some data below the quantification limit. J. Pharmacokinet. Pharmacodyn. 28:481-504. - PubMed
-
- Beal, S. L., and L. B. Sheiner. 1998. NONMEM user's guide; NONMEM project group. University of California at San Francisco, San Francisco, Calif.
-
- Bergshoeff, A. S., P. L. Fraaij, A. M. van Rossum, T. F. Wolfs, S. P. Geelen, R. de Groot, and D. M. Burger. 2003. Pharmacokinetics of nelfinavir in children: influencing factors and dose implications. Antivir. Ther. 8:215-222. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

