Artesunate suppositories versus intramuscular artemether for treatment of severe malaria in children in Papua New Guinea
- PMID: 16495259
- PMCID: PMC1426445
- DOI: 10.1128/AAC.50.3.968-974.2006
Artesunate suppositories versus intramuscular artemether for treatment of severe malaria in children in Papua New Guinea
Abstract
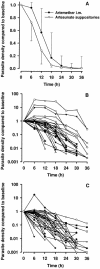
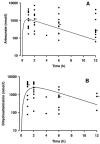
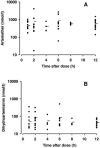
Drug treatment of severe malaria must be rapidly effective. Suppositories may be valuable for childhood malaria when circumstances prevent oral or parenteral therapy. We compared artesunate suppositories (n = 41; 8 to 16 mg/kg of body weight at 0 and 12 h and then daily) with intramuscular (i.m.) artemether (n = 38; 3.2 mg/kg at 0 h and then 1.6 mg/kg daily) in an open-label, randomized trial with children with severe Plasmodium falciparum malaria in Papua New Guinea (PNG). Parasite density and temperature were measured every 6 h for > or = 72 h. Primary endpoints included times to 50% and 90% parasite clearance (PCT50 and PCT90) and the time to per os status. In a subset of 29 patients, plasma levels of artemether, artesunate, and their common active metabolite dihydroartemisinin were measured during the first 12 h. One suppository-treated patient with multiple complications died within 2 h of admission, but the remaining 78 recovered uneventfully. Compared to the artemether-treated children, those receiving artesunate suppositories had a significantly earlier mean PCT50 (9.1 versus 13.8 h; P = 0.008) and PCT90 (15.6 versus 20.4 h; P = 0.011). Mean time to per os status was similar for each group. Plasma concentrations of primary drug plus active metabolite were significantly higher in the artesunate suppository group at 2 h postdose. The earlier initial fall in parasitemia with artesunate is clinically advantageous and mirrors higher initial plasma concentrations of active drug/metabolite. In severely ill children with malaria in PNG, artesunate suppositories were at least as effective as i.m. artemether and may, therefore, be useful in settings where parenteral therapy cannot be given.
Figures
References
-
- Artemether-Quinine Meta-Analysis Study Group. 2001. A meta-analysis using individual patient data of trials comparing artemether with quinine in the treatment of severe falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 95:637-650. - PubMed
-
- Awad, M. I., A. M. Y. Alkadru, R. H. Behrens, O. Z. Baraka, and I. B. Eltayeb. 2003. Descriptive study on the efficacy and safety of artesunate suppository in combination with other antimalarials in the treatment of severe malaria in Sudan. Am. J. Trop. Med. Hyg. 63:153-158. - PubMed
-
- Barnes, K. I., J. Mwenechanya, M. Tembo, H. McIlleron, P. I. Folb, I. Ribeiro, F. Little, M. Gomes, and M. E. Molyneux. 2004. Efficacy of rectal artesunate compared with parenteral quinine in initial treatment of moderately severe malaria in African children and adults: a randomised study. Lancet 363:1598-1605. - PubMed
-
- Benakis, A., M. Paris, L. Loutan, C. T. Plessas, and S. T. Plessas. 1996. Pharmacokinetic study of a new pharmaceutical form of artesunate (Plasmotrim-200 Rectocaps) administered in healthy volunteers by the rectal route. Jap. J. Trop. Med. Hyg. 24(Suppl. 1):39-45.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

