Susceptibility of Cryptococcus neoformans biofilms to antifungal agents in vitro
- PMID: 16495265
- PMCID: PMC1426450
- DOI: 10.1128/AAC.50.3.1021-1033.2006
Susceptibility of Cryptococcus neoformans biofilms to antifungal agents in vitro
Abstract
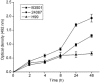
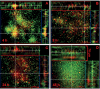
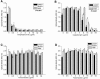
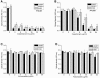
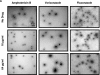
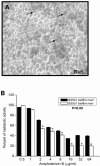
Microbial biofilms contribute to virulence and resistance to antibiotics by shielding microbial cells from host defenses and antimicrobial drugs, respectively. Cryptococcus neoformans was demonstrated to form biofilms in polystyrene microtiter plates. The numbers of CFU of disaggregated biofilms, 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide reduction, and light and confocal microscopy were used to measure the fungal mass, the metabolic activity, and the appearance of C. neoformans biofilms, respectively. Biofilm development by C. neoformans followed a standard sequence of events: fungal surface attachment, microcolony formation, and matrix production. The susceptibilities of C. neoformans cells of the biofilm and planktonic phenotypes to four antifungal agents were examined. The exposure of C. neoformans cells or preformed cryptococcal biofilms to fluconazole or voriconazole did not result in yeast growth inhibition and did not affect the metabolic activities of the biofilms, respectively. In contrast, both C. neoformans cells and preformed biofilms were susceptible to amphotericin B and caspofungin. However, C. neoformans biofilms were significantly more resistant to amphotericin B and caspofungin than planktonic cells, and their susceptibilities to these drugs were further reduced if cryptococcal cells contained melanin. A spot enzyme-linked immunosorbent assay and light and confocal microscopy were used to investigate how antifungal drugs affected C. neoformans biofilm formation. The mechanism by which amphotericin B and caspofungin interfered with C. neoformans biofilm formation involved capsular polysaccharide release and adherence. Our results suggest that biofilm formation may diminish the efficacies of some antifungal drugs during cryptococcal infection.
Figures


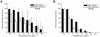
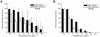
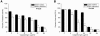

Similar articles
-
Melanization of Cryptococcus neoformans and Histoplasma capsulatum reduces their susceptibilities to amphotericin B and caspofungin.Antimicrob Agents Chemother. 2002 Nov;46(11):3394-400. doi: 10.1128/AAC.46.11.3394-3400.2002. Antimicrob Agents Chemother. 2002. PMID: 12384341 Free PMC article.
-
Specific antibody to Cryptococcus neoformans glucurunoxylomannan antagonizes antifungal drug action against cryptococcal biofilms in vitro.J Infect Dis. 2006 Jul 15;194(2):261-6. doi: 10.1086/504722. Epub 2006 Jun 14. J Infect Dis. 2006. PMID: 16779734
-
Temperature affects the susceptibility of Cryptococcus neoformans biofilms to antifungal agents.Med Mycol. 2010 Mar;48(2):421-6. doi: 10.1080/13693780903136879. Med Mycol. 2010. PMID: 19637092
-
In vitro antifungal activities of anidulafungin and micafungin, licensed agents and the investigational triazole posaconazole as determined by NCCLS methods for 12,052 fungal isolates: review of the literature.Rev Iberoam Micol. 2003 Dec;20(4):121-36. Rev Iberoam Micol. 2003. PMID: 15456349 Review.
-
Physiological Differences in Cryptococcus neoformans Strains In Vitro versus In Vivo and Their Effects on Antifungal Susceptibility.Antimicrob Agents Chemother. 2017 Feb 23;61(3):e02108-16. doi: 10.1128/AAC.02108-16. Print 2017 Mar. Antimicrob Agents Chemother. 2017. PMID: 28031206 Free PMC article. Review.
Cited by
-
Antibody-mediated immobilization of Cryptococcus neoformans promotes biofilm formation.Appl Environ Microbiol. 2009 Apr;75(8):2528-33. doi: 10.1128/AEM.02846-08. Epub 2009 Feb 27. Appl Environ Microbiol. 2009. PMID: 19251903 Free PMC article.
-
Antifungal Combination of Ethyl Acetate Extract of Poincianella pluviosa (DC.) L. P. Queiros Stem Bark With Amphotericin B in Cryptococcus neoformans.Front Microbiol. 2021 Jun 10;12:660645. doi: 10.3389/fmicb.2021.660645. eCollection 2021. Front Microbiol. 2021. PMID: 34177839 Free PMC article.
-
Magnesium Ion Acts as a Signal for Capsule Induction in Cryptococcus neoformans.Front Microbiol. 2016 Mar 15;7:325. doi: 10.3389/fmicb.2016.00325. eCollection 2016. Front Microbiol. 2016. PMID: 27014245 Free PMC article.
-
Dynamics of Mono- and Dual-Species Biofilm Formation and Interactions Between Paracoccidioides brasiliensis and Candida albicans.Front Microbiol. 2020 Oct 14;11:551256. doi: 10.3389/fmicb.2020.551256. eCollection 2020. Front Microbiol. 2020. PMID: 33178146 Free PMC article.
-
The use of chitosan to damage Cryptococcus neoformans biofilms.Biomaterials. 2010 Feb;31(4):669-79. doi: 10.1016/j.biomaterials.2009.09.087. Epub 2009 Oct 9. Biomaterials. 2010. PMID: 19819009 Free PMC article.
References
-
- Bach, M. C., P. W. Tally, and E. W. Godofsky. 1997. Use of cerebrospinal fluid shunts in patients having acquired immunodeficiency syndrome with cryptococcal meningitis and uncontrollable intracranial hypertension. Neurosurgery 41:1280-1282. - PubMed
-
- Baillie, G. S., and L. J. Douglas. 2000. Matrix polymers of Candida biofilms and their possible role in biofilm resistance to antifungal agents. J. Antimicrob. Chemother. 46:397-403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

