Caspase-2-induced apoptosis requires bid cleavage: a physiological role for bid in heat shock-induced death
- PMID: 16495337
- PMCID: PMC1446087
- DOI: 10.1091/mbc.e05-12-1107
Caspase-2-induced apoptosis requires bid cleavage: a physiological role for bid in heat shock-induced death
Abstract
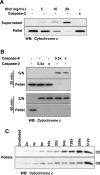
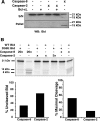
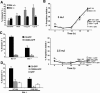
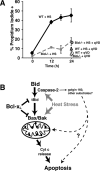
The mechanisms through which Caspase-2 leads to cell death are controversial. Here we show, using a combination of cell-free and cell culture-based approaches, that cleavage of the Bcl-2-family protein Bid is required for the induction of apoptosis by Caspase-2. Caspase-2 promoted cytochrome c release from mitochondria in the presence of cytosol from wild-type, but not Bid-deficient, mouse embryonic fibroblasts (MEFs). Recombinant wild-type Bid, but not a noncleavable mutant (D59E), restored cytochrome c release. Similarly, Bid-null MEFs were relatively resistant to apoptosis triggered by active Caspase-2, and apoptosis was restored in Bid-null cells by the expression of wild-type, but not D59E, Bid. Finally, Bid-null MEFs were substantially more resistant to apoptosis induced by heat shock, which has been shown to be dependent on apical activation of Caspase-2. The data are consistent with a model in which Caspase-2 induces apoptosis via cleavage of Bid at D59 and the subsequent engagement of the mitochondrial (intrinsic) pathway.
Figures

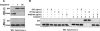
References
-
- Bossy-Wetzel, E., and Green, D. R. (1999). Caspases induce cytochrome c release from mitochondria by activating cytosolic factors. J. Biol. Chem. 274, 17484–17490. - PubMed
-
- Colussi, P. A., Harvey, N. L., and Kumar, S. (1998). Prodomain-dependent nuclear localization of the caspase-2 (Nedd2) precursor. A novel function for a caspase prodomain. J. Biol. Chem. 273, 24535–24542. - PubMed
-
- Droin, N., Bichat, F., Rebe, C., Wotawa, A., Sordet, O., Hammann, A., Bertrand, R., and Solary, E. (2001). Involvement of caspase-2 long isoform in Fas-mediated cell death of human leukemic cells. Blood 97, 1835–1844. - PubMed
-
- Duan, H., and Dixit, V. M. (1997). RAIDD is a new `death' adaptor molecule. Nature 385, 86–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

