Functional estrogen receptors in the mitochondria of breast cancer cells
- PMID: 16495339
- PMCID: PMC1446071
- DOI: 10.1091/mbc.e05-11-1013
Functional estrogen receptors in the mitochondria of breast cancer cells
Abstract
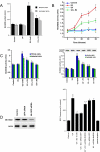
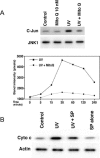
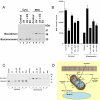
Steroid hormones have been reported to indirectly impact mitochondrial functions, attributed to nuclear receptor-induced production of proteins that localize in this cytoplasmic organelle. Here we show high-affinity estrogen receptors in the mitochondria of MCF-7 breast cancer cells and endothelial cells, compatible with classical estrogen receptors ERalpha and ERbeta. We report that in MCF-7, estrogen inhibits UV radiation-induced cytochrome C release, the decrease of the mitochondrial membrane potential, and apoptotic cell death. UV stimulated the formation of mitochondrial reactive oxygen species (mROS), and mROS were essential to inducing mitochondrial events of cell death. mROS mediated the UV activation of c-jun N-terminal kinase (JNK), and protein kinase C (PKC) delta, underlying the subsequent translocation of Bax to the mitochondria where oligomerization was promoted. E2 (estradiol) inhibited all these events, directly acting in mitochondria to inhibit mROS by rapidly up-regulating manganese superoxide dismutase activity. We implicate novel functions of ER in the mitochondria of breast cancer that lead to the survival of the tumor cells.
Figures
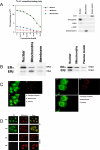
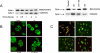
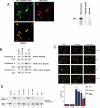
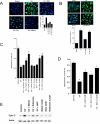

References
-
- Aslan, M., and Ozben, T. (2003). Oxidants in receptor tyrosine kinase signal transduction pathways. Antioxid. Redox. Signal 5, 781–788. - PubMed
-
- Borras, C., Sastre, J., Garcia-Sala, D., Lloret, A., Pallardo, F. V., and Vina, J. (2003). Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic. Biol. Med. 34, 546–552. - PubMed
-
- Borras, C., Gambini, J., Gomez-Cabrera, M. C., Sastre, J., Pallardo, F. V., Mann,G. E., and Vina, J. (2005).. 17beta-oestradiol up-regulates longevity-related, antioxidant enzyme expression via the ERK1 and ERK2[MAPK]/NFkappaB cascade. Aging Cell 4, 113–118. - PubMed
-
- Brodie, C., and Blumberg, P. M. (2003). Regulation of cell apoptosis by protein kinase c delta. Apoptosis 8, 19–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

