Serotonergic modulation of inspiratory hypoglossal motoneurons in decerebrate dogs
- PMID: 16495364
- PMCID: PMC2582383
- DOI: 10.1152/jn.00823.2005
Serotonergic modulation of inspiratory hypoglossal motoneurons in decerebrate dogs
Abstract
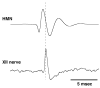
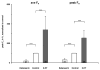
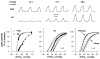
Inspiratory hypoglossal motoneurons (IHMNs) maintain upper airway patency. However, this may be compromised during sleep and by sedatives, potent analgesics, and volatile anesthetics by either depression of excitatory or enhancement of inhibitory inputs. In vitro data suggest that serotonin (5-HT), through the 5-HT2A receptor subtype, plays a key role in controlling the excitability of IHMNs. We hypothesized that in vivo 5-HT modulates IHMNs activity through the 5-HT2A receptor subtype. To test this hypothesis, we used multibarrel micropipettes for extracellular single neuron recording and pressure picoejection of 5-HT or ketanserin, a selective 5-HT2A receptor subtype antagonist, onto single IHMNs in decerebrate, vagotomized, paralyzed, and mechanically ventilated dogs. Drug-induced changes in neuronal discharge frequency (F(n)) and neuronal discharge pattern were analyzed using cycle-triggered histograms. 5-HT increased the control peak F(n) to 256% and the time-averaged F(n) to 340%. 5-HT increased the gain of the discharge pattern by 61% and the offset by 34 Hz. Ketanserin reduced the control peak F(n) by 68%, the time-averaged F(n) by 80%, and the gain by 63%. These results confirm our hypothesis that in vivo 5-HT is a potent modulator of IHMN activity through the 5-HT2A receptor subtype. Application of exogenous 5-HT shows that this mechanism is not saturated during hypercapnic hyperoxia. The two different mechanisms, gain modulation and offset change, indicate that 5-HT affects the excitability as well as the excitation of IHMNs in vivo.
Figures


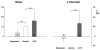


References
-
- Aldes LD, Chapman ME, Chronister RB, Haycock JW. Sources of noradrenergic afferents to the hypoglossal nucleus in the rat. Brain Res Bull. 1992;29:931–942. - PubMed
-
- Anderson JW, Sant'Ambrogio FB, Orani GP, Sant'Ambrogio G, Mathew OP. Carbon dioxide-responsive laryngeal receptors in the dog. Respir Physiol. 1990;82:217–226. - PubMed
-
- Bayliss DA, Viana F, Talley EM, Berger AJ. Neuromodulation of hypoglossal motoneurons: cellular and developmental mechanisms. Respir Physiol. 1997;110:139–150. - PubMed
-
- Berger AJ, Bayliss DA, Viana F. Modulation of neonatal rat hypoglossal motoneuron excitability by serotonin. Neurosci Lett. 1992;143:164–168. - PubMed
-
- Brandes IF, Dean C, Jakovcevic D, Stucke AG, Hopp FA, Zuperku EJ, Stuth EA. Anatomical location of genioglossus motoneurons within the adult canine hypoglossal motor nucleus. FASEB J. 2004a;D 561:468.7.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

