The kinetics of synaptic vesicle reacidification at hippocampal nerve terminals
- PMID: 16495458
- PMCID: PMC6674811
- DOI: 10.1523/JNEUROSCI.4425-05.2006
The kinetics of synaptic vesicle reacidification at hippocampal nerve terminals
Abstract
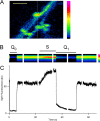
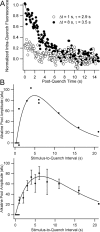
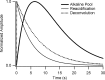
After exocytosis, synaptic vesicles are recycled locally in the synaptic terminal and are refilled with neurotransmitter via vesicular transporters. The biophysical mechanisms of refilling are poorly understood, but it is clear that the generation of a proton gradient across the vesicle membrane is crucial. To better understand the determinants of vesicle refilling, we developed a novel method to measure unambiguously the kinetics of synaptic vesicle reacidification at individual synaptic terminals. Hippocampal neurons transfected with synapto-pHluorin (SpH), a synaptic vesicle-targeted lumenal GFP (green fluorescent protein), whose fluorescence is quenched when protonated (pKa approximately 7.1), were rapidly surface-quenched immediately after trains of repetitive electrical stimulation. The recently endocytosed alkaline pool of SpH is protected from such surface quenching, and its fluorescence decay reflects reacidification kinetics. These measurements indicate that, after compensatory endocytosis, synaptic vesicles reacidify with first-order kinetics (tau approximately 4-5 s) and that their rate of reacidification is subject to slowing by increased external buffer.
Figures


References
-
- Aravanis AM, Pyle JL, Tsien RW (2003). Single synaptic vesicles fusing transiently and successively without loss of identity. Nature 423:643–647. - PubMed
-
- Fernandez-Alfonso T, Ryan TA (2004). The kinetics of synaptic vesicle pool depletion at CNS synaptic terminals. Neuron 41:943–953. - PubMed
-
- Fremeau RT Jr, Voglmaier S, Seal RP, Edwards RH (2004). VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci 27:98–103. - PubMed
-
- Gandhi SP, Stevens CF (2003). Three modes of synaptic vesicular recycling revealed by single-vesicle imaging. Nature 423:607–613. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
