Transsynaptic signaling by postsynaptic synapse-associated protein 97
- PMID: 16495462
- PMCID: PMC6674804
- DOI: 10.1523/JNEUROSCI.5247-05.2006
Transsynaptic signaling by postsynaptic synapse-associated protein 97
Abstract
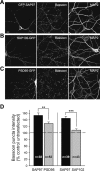
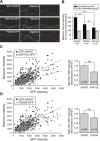
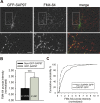
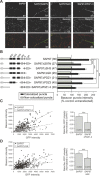
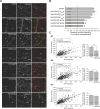
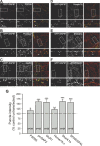
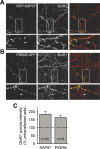
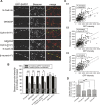
The molecular mechanisms by which postsynaptic modifications lead to precisely coordinated changes in presynaptic structure and function are primarily unknown. To address this issue, we examined the presynaptic consequences of postsynaptic expression of members of the membrane-associated guanylate kinase family of synaptic scaffolding proteins. Postsynaptic expression of synapse-associated protein 97 (SAP97) increased presynaptic protein content and active zone size to a greater extent than comparable amounts of postsynaptic PSD-95 (postsynaptic density-95) or SAP102. In addition, postsynaptic expression of SAP97 enhanced presynaptic function, as measured by increased FM4-64 dye uptake. The structural presynaptic effects of postsynaptic SAP97 required ligand binding through two of its PDZ (PSD-95/Discs large/zona occludens-1) domains as well as intact N-terminal and guanylate kinase domains. Expression of SAP97 recruited a complex of additional postsynaptic proteins to synapses including glutamate receptor 1, Shank1a, SPAR (spine-associated RapGAP), and proSAP2. Furthermore, inhibition of several different transsynaptic signaling proteins including cadherins, integrins, and EphB receptor/ephrinB significantly reduced the presynaptic growth caused by postsynaptic SAP97. These results suggest that SAP97 may play a central role in the coordinated growth of synapses during development and plasticity by recruiting a complex of postsynaptic proteins that enhances presynaptic terminal growth and function via multiple transsynaptic molecular interactions.
Figures

Similar articles
-
N-terminal SAP97 isoforms differentially regulate synaptic structure and postsynaptic surface pools of AMPA receptors.Hippocampus. 2017 Jun;27(6):668-682. doi: 10.1002/hipo.22723. Epub 2017 Mar 20. Hippocampus. 2017. PMID: 28244171
-
Postsynaptic density scaffold SAP102 regulates cortical synapse development through EphB and PAK signaling pathway.J Neurosci. 2013 Mar 13;33(11):5040-52. doi: 10.1523/JNEUROSCI.2896-12.2013. J Neurosci. 2013. PMID: 23486974 Free PMC article.
-
Synapse-associated protein-97 isoform-specific regulation of surface AMPA receptors and synaptic function in cultured neurons.J Neurosci. 2003 Jun 1;23(11):4567-76. doi: 10.1523/JNEUROSCI.23-11-04567.2003. J Neurosci. 2003. PMID: 12805297 Free PMC article.
-
MAGUKs, synaptic development, and synaptic plasticity.Neuroscientist. 2011 Oct;17(5):493-512. doi: 10.1177/1073858410386384. Epub 2011 Apr 15. Neuroscientist. 2011. PMID: 21498811 Free PMC article. Review.
-
Quantification of postsynaptic density proteins: glutamate receptor subunits and scaffolding proteins.Hippocampus. 2012 May;22(5):942-53. doi: 10.1002/hipo.20950. Epub 2011 May 18. Hippocampus. 2012. PMID: 21594948 Review.
Cited by
-
Essential cooperation of N-cadherin and neuroligin-1 in the transsynaptic control of vesicle accumulation.Proc Natl Acad Sci U S A. 2010 Jun 15;107(24):11116-21. doi: 10.1073/pnas.0914233107. Epub 2010 Jun 1. Proc Natl Acad Sci U S A. 2010. PMID: 20534458 Free PMC article.
-
Transcellular Nanoalignment of Synaptic Function.Neuron. 2017 Nov 1;96(3):680-696. doi: 10.1016/j.neuron.2017.10.006. Neuron. 2017. PMID: 29096080 Free PMC article. Review.
-
A role for RhoB in synaptic plasticity and the regulation of neuronal morphology.J Neurosci. 2010 Mar 3;30(9):3508-17. doi: 10.1523/JNEUROSCI.5386-09.2010. J Neurosci. 2010. PMID: 20203211 Free PMC article.
-
Synaptic SAP97 isoforms regulate AMPA receptor dynamics and access to presynaptic glutamate.J Neurosci. 2009 Apr 8;29(14):4332-45. doi: 10.1523/JNEUROSCI.4431-08.2009. J Neurosci. 2009. PMID: 19357261 Free PMC article.
-
Synchronous and asynchronous transmitter release at nicotinic synapses are differentially regulated by postsynaptic PSD-95 proteins.J Neurosci. 2009 Dec 16;29(50):15770-9. doi: 10.1523/JNEUROSCI.4951-09.2009. J Neurosci. 2009. PMID: 20016093 Free PMC article.
References
-
- Belichenko PV, Masliah E, Kleschevnikov AM, Villar AJ, Epstein CJ, Salehi A, Mobley WC (2004). Synaptic structural abnormalities in the Ts65Dn mouse model of Down syndrome. J Comp Neurol 480:281–298. - PubMed
-
- Betz WJ, Bewick GS (1992). Optical analysis of synaptic vesicle recycling at the frog neuromuscular junction. Science 255:200–203. - PubMed
-
- Boeckers TM, Winter C, Smalla KH, Kreutz MR, Bockmann J, Seidenbecher C, Garner CC, Gundelfinger ED (1999). Proline-rich synapse-associated proteins ProSAP1 and ProSAP2 interact with synaptic proteins of the SAPAP/GKAP family. Biochem Biophys Res Commun 264:247–252. - PubMed
-
- Boeckers TM, Bockmann J, Kreutz MR, Gundelfinger ED (2002). ProSAP/Shank proteins–a family of higher order organizing molecules of the postsynaptic density with an emerging role in human neurological disease. J Neurochem 81:903–910. - PubMed
-
- Cai C, Coleman SK, Niemi K, Keinanen K (2002). Selective binding of synapse-associated protein 97 to GluR-A alpha-amino-5-hydroxy-3-methyl-4-isoxazole propionate receptor subunit is determined by a novel sequence motif. J Biol Chem 277:31484–31490. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
