Mutual enhancement of virulence by enterotoxigenic and enteropathogenic Escherichia coli
- PMID: 16495521
- PMCID: PMC1418639
- DOI: 10.1128/IAI.74.3.1505-1515.2006
Mutual enhancement of virulence by enterotoxigenic and enteropathogenic Escherichia coli
Abstract
Enterotoxigenic Escherichia coli (ETEC) and enteropathogenic E. coli (EPEC) are common causes of diarrhea in children in developing countries. Dual infections with both pathogens have been noted fairly frequently in studies of diarrhea around the world. In previous laboratory work, we noted that cholera toxin and forskolin markedly potentiated EPEC-induced ATP release from the host cell, and this potentiated release was found to be mediated by the cystic fibrosis transmembrane conductance regulator. In this study, we examined whether the ETEC heat-labile toxin (LT) or the heat-stable toxin (STa, also known as ST) potentiated EPEC-induced ATP release. We found that crude ETEC culture filtrates, as well as purified ETEC toxins, did potentiate EPEC-induced ATP release in cultured T84 cells. Coinfection of T84 cells with live ETEC plus EPEC bacteria also resulted in enhanced ATP release compared to EPEC alone. In Ussing chamber studies of chloride secretion, adenine nucleotides released from the host by EPEC also significantly enhanced the chloride secretory responses that were triggered by crude ETEC filtrates, purified STa, and the peptide hormone guanylin. In addition, adenosine and LT had additive or synergistic effects in inducing vacuole formation in T84 cells. Therefore, ETEC toxins and EPEC-induced damage to the host cell both enhance the virulence of the other type of E. coli. Our in vitro data demonstrate a molecular basis for a microbial interaction, which could result in increased severity of disease in vivo in individuals who are coinfected with ETEC and EPEC.
Figures
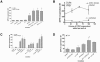
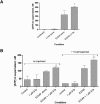
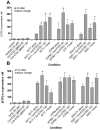




Similar articles
-
Release of ATP during host cell killing by enteropathogenic E. coli and its role as a secretory mediator.Am J Physiol Gastrointest Liver Physiol. 2002 Jul;283(1):G74-86. doi: 10.1152/ajpgi.00484.2001. Am J Physiol Gastrointest Liver Physiol. 2002. PMID: 12065294
-
Immunological cross-reactivity of heat-labile enterotoxins produced by enterotoxigenic and enteropathogenic strains of Escherichia coli.Immunology. 1980 Sep;41(1):115-21. Immunology. 1980. PMID: 7000692 Free PMC article.
-
Two pathways for ATP release from host cells in enteropathogenic Escherichia coli infection.Am J Physiol Gastrointest Liver Physiol. 2005 Sep;289(3):G407-17. doi: 10.1152/ajpgi.00137.2005. Am J Physiol Gastrointest Liver Physiol. 2005. PMID: 16093420
-
Escherichia coli as a pathogen in dogs and cats.Vet Res. 1999 Mar-Jun;30(2-3):285-98. Vet Res. 1999. PMID: 10367359 Review.
-
Enterotoxins, colonization factors and serotypes of enterotoxigenic Escherichia coli from humans and animals.Microbiologia. 1991 Sep;7(2):57-73. Microbiologia. 1991. PMID: 1684712 Review.
Cited by
-
Enterotoxigenic Escherichia coli heat labile enterotoxin inhibits intestinal ascorbic acid uptake via a cAMP-dependent NF-κB-mediated pathway.Am J Physiol Gastrointest Liver Physiol. 2019 Jan 1;316(1):G55-G63. doi: 10.1152/ajpgi.00259.2018. Epub 2018 Oct 4. Am J Physiol Gastrointest Liver Physiol. 2019. PMID: 30285481 Free PMC article.
-
Feedback effects of host-derived adenosine on enteropathogenic Escherichia coli.FEMS Immunol Med Microbiol. 2009 Dec;57(3):214-28. doi: 10.1111/j.1574-695X.2009.00598.x. Epub 2009 Aug 18. FEMS Immunol Med Microbiol. 2009. PMID: 19751218 Free PMC article.
-
Concomitant enterotoxigenic Escherichia coli infection induces increased immune responses to Vibrio cholerae O1 antigens in patients with cholera in Bangladesh.Infect Immun. 2010 May;78(5):2117-24. doi: 10.1128/IAI.01426-09. Epub 2010 Feb 22. Infect Immun. 2010. PMID: 20176796 Free PMC article.
-
Effect of zinc in enteropathogenic Escherichia coli infection.Infect Immun. 2007 Dec;75(12):5974-84. doi: 10.1128/IAI.00750-07. Epub 2007 Sep 17. Infect Immun. 2007. PMID: 17875638 Free PMC article.
-
Thiophenecarboxylate suppressor of cyclic nucleotides discovered in a small-molecule screen blocks toxin-induced intestinal fluid secretion.Mol Pharmacol. 2009 Jan;75(1):134-42. doi: 10.1124/mol.108.050567. Epub 2008 Sep 29. Mol Pharmacol. 2009. PMID: 18824527 Free PMC article.
References
-
- Adhikari, M., Y. Coovadi, and J. Hewitt. 1985. Enteropathogenic Escherichia coli (EPEC) and enterotoxigenic (ETEC) related diarrhoeal disease in a neonatal unit. Ann. Trop. Paediatr. 5:19-22. - PubMed
-
- Barkla, D. H., R. H. Whitehead, and I. P. Hayward. 1992. Effects of cholera toxin on human colon carcinoma cell lines. Pathology 24:296-301. - PubMed
-
- Bieber, D., S. Ramer, C.-Y. Wu, W. Murray, T. Tobe, R. Fernandez, and G. Schoolnik. 1998. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science 280:2114-2118. - PubMed
-
- Braunstein, G. M., R. M. Roman, J. P. Clancy, B. A. Kudlow, A. L. Taylor, V. G. Shylonsky, B. Jovov, K. Peter, T. Jilling, I. I. Ismailov, D. J. Benos, L. M. Schwiebert, J. G. Fitz, and E. M. Schwiebert. 2001. Cystic fibrosis transmembrane conductance regulator facilitates ATP release by stimulating a separate ATP release channel for autocrine control of cell volume regulation. J. Biol. Chem. 276:6621-6630. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

