Binding of vitronectin by the Moraxella catarrhalis UspA2 protein interferes with late stages of the complement cascade
- PMID: 16495531
- PMCID: PMC1418666
- DOI: 10.1128/IAI.74.3.1597-1611.2006
Binding of vitronectin by the Moraxella catarrhalis UspA2 protein interferes with late stages of the complement cascade
Abstract
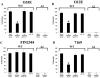
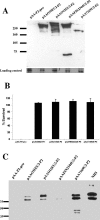
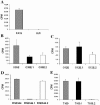
Many Moraxella catarrhalis strains are resistant to the bactericidal activity of normal human serum (NHS). The UspA2 protein of the serum-resistant strain O35E has previously been shown to be directly involved in conferring serum resistance on this strain. Testing of 11 additional serum-resistant M. catarrhalis wild-type isolates and their uspA1 and uspA2 mutants showed that the uspA1 mutants of all 11 strains were consistently serum resistant and that the uspA2 mutants of these same 11 strains were always serum sensitive. Analysis of complement deposition on four different serum-resistant M. catarrhalis strains and their serum-sensitive uspA2 mutants showed that, for three of these four strain sets, the wild-type and mutant strains bound similar amounts of early complement components. In contrast, there was a significant reduction in the amount of the polymerized C9 on the wild-type strains relative to that on the uspA2 mutants. These same three wild-type strains bound more vitronectin than did their uspA2 mutants. UspA2 proteins from these three strains, when expressed in Haemophilus influenzae, bound vitronectin and conferred serum resistance on this organism. Furthermore, vitronectin-depleted NHS exhibited bactericidal activity against these same three serum-resistant wild-type strains; addition of purified vitronectin to this serum restored serum resistance. In contrast, binding of the complement regulator C4b-binding protein by the M. catarrhalis strains used in this study was found to be highly variable and did not appear to correlate with the serum-resistant phenotype. These results indicate that binding of vitronectin by UspA2 is involved in the serum resistance of M. catarrhalis; this represents the first example of vitronectin-mediated serum resistance on a microbe.
Figures




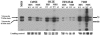


References
-
- Alexander, H. E. 1965. The Haemophilus group, p. 724-741. In R. J. Dubos and J. G. Hirsch (ed.), Bacterial and mycotic infections of man. J. B. Lippincott Co., Philadelphia, Pa.
-
- Apicella, M. A., R. E. Mandrell, M. Shero, M. E. Wilson, J. M. Griffiss, G. F. Brooks, C. Lammel, J. F. Breen, and P. A. Rice. 1990. Modification by sialic acid of Neisseria gonorrhoeae lipooligosaccharide epitope expression in human urethral exudates: an immunoelectron microscopic analysis. J. Infect. Dis. 162:506-512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

