Vaccination of mice with gonococcal TbpB expressed in vivo from Venezuelan equine encephalitis viral replicon particles
- PMID: 16495532
- PMCID: PMC1418633
- DOI: 10.1128/IAI.74.3.1612-1620.2006
Vaccination of mice with gonococcal TbpB expressed in vivo from Venezuelan equine encephalitis viral replicon particles
Abstract
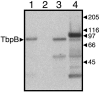
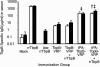
We investigated the immunogenicity of gonococcal transferrin binding protein B (TbpB) expressed with and without a eukaryotic secretion signal from a nonpropagating Venezuelan equine encephalitis virus replicon particle (VRP) delivery system. TbpB was successfully expressed in baby hamster kidney (BHK) cells, and the presence of the eukaryotic secretion signal not only apparently increased the protein's expression but also allowed for extracellular localization and glycosylation. Mice immunized with VRPs produced significant amounts of serum antibody although less than the amounts produced by mice immunized with recombinant protein. The response of mice immunized with VRPs encoding TbpB was consistently more Th1 biased than the response of mice immunized with recombinant protein alone. Boosting with recombinant protein following immunization with TbpB VRPs resulted in higher specific-antibody levels without altering the Th1/Th2 bias. Most of the immunization groups produced significant specific antibody binding to the intact surface of the homologous Neisseria gonorrhoeae strain. Immunization with TbpB VRPs without a eukaryotic secretion signal generated no measurable specific antibodies on the genital mucosal surface, but inclusion of a eukaryotic secretion signal or boosting with recombinant protein resulted in specific immunoglobulin G (IgG) and IgA in mucosal secretions after TbpB VRP immunization. The TbpB VRP system has potential for an N. gonorrhoeae vaccine.
Figures


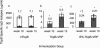



Similar articles
-
Comparison of immune responses to gonococcal PorB delivered as outer membrane vesicles, recombinant protein, or Venezuelan equine encephalitis virus replicon particles.Infect Immun. 2005 Nov;73(11):7558-68. doi: 10.1128/IAI.73.11.7558-7568.2005. Infect Immun. 2005. PMID: 16239559 Free PMC article.
-
Intranasal administration of recombinant Neisseria gonorrhoeae transferrin binding proteins A and B conjugated to the cholera toxin B subunit induces systemic and vaginal antibodies in mice.Infect Immun. 2005 Jul;73(7):3945-53. doi: 10.1128/IAI.73.7.3945-3953.2005. Infect Immun. 2005. PMID: 15972481 Free PMC article.
-
DNA immunization of mice with a plasmid encoding Neisseria gonorrhea PorB protein by intramuscular injection and epidermal particle bombardment.Vaccine. 2004 Jan 26;22(5-6):660-9. doi: 10.1016/j.vaccine.2003.08.036. Vaccine. 2004. PMID: 14741158
-
Alphavirus replicon particles as candidate HIV vaccines.IUBMB Life. 2002 Apr-May;53(4-5):209-11. doi: 10.1080/15216540212657. IUBMB Life. 2002. PMID: 12120997 Review.
-
Progress Toward a Gonococcal Vaccine: The Way Forward.Front Immunol. 2019 Oct 15;10:2417. doi: 10.3389/fimmu.2019.02417. eCollection 2019. Front Immunol. 2019. PMID: 31681305 Free PMC article. Review.
Cited by
-
Identification of TbpA residues required for transferrin-iron utilization by Neisseria gonorrhoeae.Infect Immun. 2008 May;76(5):1960-9. doi: 10.1128/IAI.00020-08. Epub 2008 Mar 17. Infect Immun. 2008. PMID: 18347046 Free PMC article.
-
Neisseria gonorrhoeae vaccines: a contemporary overview.Clin Microbiol Rev. 2024 Mar 14;37(1):e0009423. doi: 10.1128/cmr.00094-23. Epub 2024 Jan 16. Clin Microbiol Rev. 2024. PMID: 38226640 Free PMC article. Review.
-
Conserved regions of gonococcal TbpB are critical for surface exposure and transferrin iron utilization.Infect Immun. 2013 Sep;81(9):3442-50. doi: 10.1128/IAI.00280-13. Epub 2013 Jul 8. Infect Immun. 2013. PMID: 23836816 Free PMC article.
-
Enhancement of protein expression by alphavirus replicons by designing self-replicating subgenomic RNAs.Proc Natl Acad Sci U S A. 2014 Jul 22;111(29):10708-13. doi: 10.1073/pnas.1408677111. Epub 2014 Jul 7. Proc Natl Acad Sci U S A. 2014. PMID: 25002490 Free PMC article.
-
Addressing Sexually Transmitted Infections Due to Neisseria gonorrhoeae in the Present and Future.Microorganisms. 2024 Apr 28;12(5):884. doi: 10.3390/microorganisms12050884. Microorganisms. 2024. PMID: 38792714 Free PMC article. Review.
References
-
- Balasuriya, U. B., H. W. Heidner, N. L. Davis, H. M. Wagner, P. J. Hullinger, J. F. Hedges, J. C. Williams, R. E. Johnston, W. D. Wilson, I. K. Liu, and N. J. MacLachlan. 2002. Alphavirus replicon particles expressing the two major envelope proteins of equine arteritis virus induce high level protection against challenge with virulent virus in vaccinated horses. Vaccine 20:1609-1617. - PubMed
-
- Balasuriya, U. B., H. W. Heidner, J. F. Hedges, J. C. Williams, N. L. Davis, R. E. Johnston, and N. J. MacLachlan. 2000. Expression of the two major envelope proteins of equine arteritis virus as a heterodimer is necessary for induction of neutralizing antibodies in mice immunized with recombinant Venezuelan equine encephalitis virus replicon particles. J. Virol. 74:10623-10630. - PMC - PubMed
-
- Bszewczyki, A., and L. M. Koloft. 1985. A method for efficient blotting of basic proteins from SDS-PAGE to nitrocellulose. Anal. Biochem. 150:403-407. - PubMed
-
- Cassetti, M. C., S. P. McElhiney, V. Shahabi, J. K. Pullen, I. C. Le Poole, G. L. Eiben, L. R. Smith, and W. M. Kast. 2004. Antitumor efficacy of Venezuelan equine encephalitis virus replicon particles encoding mutated HPV16 E6 and E7 genes. Vaccine 22:520-527. - PubMed
-
- Cohen, M. S., J. G. Cannon, A. E. Jerse, L. M. Charniga, S. F. Isbey, and L. G. Whicker. 1994. Human experimentation with Neisseria gonorrhoeae: rationale, methods, and implications for the biology of infection and vaccine development. J. Infect. Dis. 169:532-537. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

