Vaccination of mice with gonococcal TbpB expressed in vivo from Venezuelan equine encephalitis viral replicon particles
- PMID: 16495532
- PMCID: PMC1418633
- DOI: 10.1128/IAI.74.3.1612-1620.2006
Vaccination of mice with gonococcal TbpB expressed in vivo from Venezuelan equine encephalitis viral replicon particles
Abstract
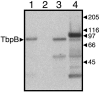
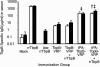
We investigated the immunogenicity of gonococcal transferrin binding protein B (TbpB) expressed with and without a eukaryotic secretion signal from a nonpropagating Venezuelan equine encephalitis virus replicon particle (VRP) delivery system. TbpB was successfully expressed in baby hamster kidney (BHK) cells, and the presence of the eukaryotic secretion signal not only apparently increased the protein's expression but also allowed for extracellular localization and glycosylation. Mice immunized with VRPs produced significant amounts of serum antibody although less than the amounts produced by mice immunized with recombinant protein. The response of mice immunized with VRPs encoding TbpB was consistently more Th1 biased than the response of mice immunized with recombinant protein alone. Boosting with recombinant protein following immunization with TbpB VRPs resulted in higher specific-antibody levels without altering the Th1/Th2 bias. Most of the immunization groups produced significant specific antibody binding to the intact surface of the homologous Neisseria gonorrhoeae strain. Immunization with TbpB VRPs without a eukaryotic secretion signal generated no measurable specific antibodies on the genital mucosal surface, but inclusion of a eukaryotic secretion signal or boosting with recombinant protein resulted in specific immunoglobulin G (IgG) and IgA in mucosal secretions after TbpB VRP immunization. The TbpB VRP system has potential for an N. gonorrhoeae vaccine.
Figures


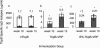



References
-
- Balasuriya, U. B., H. W. Heidner, N. L. Davis, H. M. Wagner, P. J. Hullinger, J. F. Hedges, J. C. Williams, R. E. Johnston, W. D. Wilson, I. K. Liu, and N. J. MacLachlan. 2002. Alphavirus replicon particles expressing the two major envelope proteins of equine arteritis virus induce high level protection against challenge with virulent virus in vaccinated horses. Vaccine 20:1609-1617. - PubMed
-
- Balasuriya, U. B., H. W. Heidner, J. F. Hedges, J. C. Williams, N. L. Davis, R. E. Johnston, and N. J. MacLachlan. 2000. Expression of the two major envelope proteins of equine arteritis virus as a heterodimer is necessary for induction of neutralizing antibodies in mice immunized with recombinant Venezuelan equine encephalitis virus replicon particles. J. Virol. 74:10623-10630. - PMC - PubMed
-
- Bszewczyki, A., and L. M. Koloft. 1985. A method for efficient blotting of basic proteins from SDS-PAGE to nitrocellulose. Anal. Biochem. 150:403-407. - PubMed
-
- Cassetti, M. C., S. P. McElhiney, V. Shahabi, J. K. Pullen, I. C. Le Poole, G. L. Eiben, L. R. Smith, and W. M. Kast. 2004. Antitumor efficacy of Venezuelan equine encephalitis virus replicon particles encoding mutated HPV16 E6 and E7 genes. Vaccine 22:520-527. - PubMed
-
- Cohen, M. S., J. G. Cannon, A. E. Jerse, L. M. Charniga, S. F. Isbey, and L. G. Whicker. 1994. Human experimentation with Neisseria gonorrhoeae: rationale, methods, and implications for the biology of infection and vaccine development. J. Infect. Dis. 169:532-537. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

