Multilevel control of competence development and stress tolerance in Streptococcus mutans UA159
- PMID: 16495534
- PMCID: PMC1418624
- DOI: 10.1128/IAI.74.3.1631-1642.2006
Multilevel control of competence development and stress tolerance in Streptococcus mutans UA159
Abstract
Genetic competence appears to be important in establishment of biofilms and tolerance of environmental insults. We report here that the development of competence is controlled at multiple levels in a complex network that includes two signal-transducing two-component systems (TCS). Using Streptococcus mutans strain UA159, we demonstrate that the histidine kinase CiaH, but not the response regulator CiaR, causes a dramatic decrease in biofilm formation and in transformation efficiency. Inactivation of comE or comD had no effect on stress tolerance, but transformability of the mutants was poor and was not restored by addition of competence-stimulating peptide (CSP). Horse serum (HS) or bovine serum albumin (BSA) had no impact on transformability of any strains. Interestingly, though, the presence of HS or BSA in combination with CSP was required for efficient induction of comD, comX, and comYA, and induction was dependent on ComDE and CiaH, but not CiaR. Inactivation of comC, encoding CSP, had no impact on transformation, and CiaH was shown to be required for optimal comC expression. This study reveals that S. mutans integrates multiple environmental signals through CiaHR and ComDE to coordinate induction of com genes and that CiaH can exert its influence through CiaR and as-yet-unidentified regulators. The results highlight critical differences in the role and regulation of CiaRH and com genes in different S. mutans isolates and between S. mutans and Streptococcus pneumoniae, indicating that substantial divergence in the role and regulation of TCS and competence genes has occurred in streptococci.
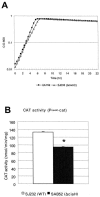
Figures






References
-
- Alloing, G., B. Martin, C. Granadel, and J. P. Claverys. 1998. Development of competence in Streptococcus pneumonaie: pheromone autoinduction and control of quorum sensing by the oligopeptide permease. Mol. Microbiol. 29:75-83. - PubMed
-
- Berge, M., M. Moscoso, M. Prudhomme, B. Martin, and J. P. Claverys. 2002. Uptake of transforming DNA in gram-positive bacteria: a view from Streptococcus pneumoniae. Mol. Microbiol. 45:411-421. - PubMed
-
- Burne, R. A. 1998. Oral streptococci: products of their environment. J. Dent. Res. 77:445-452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

