Gingipains of Porphyromonas gingivalis modulate leukocyte adhesion molecule expression induced in human endothelial cells by ligation of CD99
- PMID: 16495537
- PMCID: PMC1418641
- DOI: 10.1128/IAI.74.3.1661-1672.2006
Gingipains of Porphyromonas gingivalis modulate leukocyte adhesion molecule expression induced in human endothelial cells by ligation of CD99
Abstract
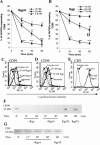
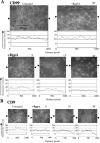
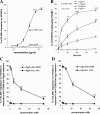
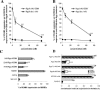
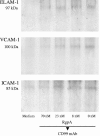
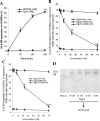
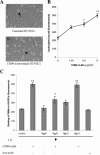
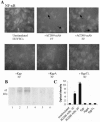
Porphyromonas gingivalis has been implicated as a key etiologic agent in the pathogenesis of destructive chronic periodontitis. Among virulence factors of this organism are cysteine proteinases, or gingipains, that have the capacity to modulate host inflammatory defenses. Intercellular adhesion molecule expression by vascular endothelium represents a crucial process for leukocyte transendothelial migration into inflamed tissue. Ligation of CD99 on endothelial cells was shown to induce expression of endothelial leukocyte adhesion molecule 1, vascular cell adhesion molecule 1, intercellular adhesion molecule 1, and major histocompatibility complex class II molecules and to increase adhesion of leukocytes. CD99 ligation was also found to induce nuclear translocation of NF-kappaB. These results indicate that endothelial cell activation by CD99 ligation may lead to the up-regulation of adhesion molecule expression via NF-kappaB activation. However, pretreatment of endothelial cells with gingipains caused a dose-dependent reduction of adhesion molecule expression and leukocyte adhesion induced by ligation of CD99 on endothelial cells. The data provide evidence that the gingipains can reduce the functional expression of CD99 on endothelial cells, leading indirectly to the disruption of adhesion molecule expression and of leukocyte recruitment to inflammatory foci.
Figures
Similar articles
-
Functional implication of the hydrolysis of platelet endothelial cell adhesion molecule 1 (CD31) by gingipains of Porphyromonas gingivalis for the pathology of periodontal disease.Infect Immun. 2005 Mar;73(3):1386-98. doi: 10.1128/IAI.73.3.1386-1398.2005. Infect Immun. 2005. PMID: 15731036 Free PMC article.
-
Porphyromonas gingivalis Gingipains Destroy the Vascular Barrier and Reduce CD99 and CD99L2 Expression To Regulate Transendothelial Migration.Microbiol Spectr. 2023 Jun 15;11(3):e0476922. doi: 10.1128/spectrum.04769-22. Epub 2023 May 18. Microbiol Spectr. 2023. PMID: 37199607 Free PMC article.
-
Gingipains from Porphyromonas gingivalis W83 synergistically disrupt endothelial cell adhesion and can induce caspase-independent apoptosis.Infect Immun. 2006 Oct;74(10):5667-78. doi: 10.1128/IAI.01140-05. Infect Immun. 2006. PMID: 16988242 Free PMC article.
-
The role of gingipains in the pathogenesis of periodontal disease.J Periodontol. 2003 Jan;74(1):111-8. doi: 10.1902/jop.2003.74.1.111. J Periodontol. 2003. PMID: 12593605 Review.
-
Active participation of endothelial cells in inflammation.J Leukoc Biol. 2005 Apr;77(4):487-95. doi: 10.1189/jlb.0904554. Epub 2005 Jan 3. J Leukoc Biol. 2005. PMID: 15629883 Free PMC article. Review.
Cited by
-
Local and Systemic Effects of Porphyromonas gingivalis Infection.Microorganisms. 2023 Feb 13;11(2):470. doi: 10.3390/microorganisms11020470. Microorganisms. 2023. PMID: 36838435 Free PMC article. Review.
-
Association of CD99 short and long forms with MHC class I, MHC class II and tetraspanin CD81 and recruitment into immunological synapses.BMC Res Notes. 2011 Aug 13;4:293. doi: 10.1186/1756-0500-4-293. BMC Res Notes. 2011. PMID: 21838920 Free PMC article.
-
Gingipain-dependent interactions with the host are important for survival of Porphyromonas gingivalis.Front Biosci. 2008 May 1;13:3215-38. doi: 10.2741/2922. Front Biosci. 2008. PMID: 18508429 Free PMC article. Review.
-
Vascular Changes and Hypoxia in Periodontal Disease as a Link to Systemic Complications.Pathogens. 2021 Oct 5;10(10):1280. doi: 10.3390/pathogens10101280. Pathogens. 2021. PMID: 34684229 Free PMC article. Review.
-
Dichotomy of gingipains action as virulence factors: from cleaving substrates with the precision of a surgeon's knife to a meat chopper-like brutal degradation of proteins.Periodontol 2000. 2010 Oct;54(1):15-44. doi: 10.1111/j.1600-0757.2010.00377.x. Periodontol 2000. 2010. PMID: 20712631 Free PMC article. Review. No abstract available.
References
-
- Allison, J. P., and L. L. Lanier. 1987. Structure, function and serology of the T-cell antigen receptor complex. Annu. Rev. Immunol. 5:503-540. - PubMed
-
- Amar, S., N. Gokce, S. Morgan, M. Loukideli, T. E. Van Dyke, and J. A. Vita. 2003. Periodontal disease is associated with brachial artery endothelial dysfunction and systemic inflammation. Arterioscler. Thromb. Vasc. Biol. 23:1245-1249. - PubMed
-
- Aubrit, F., C. Gelin, D. Pham, B. Raynal, and A. Bernard. 1989. The biochemical characterization of E2, a T cell surface molecule involved in rosettes. Eur. J. Immunol. 19:1431-1436. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

