Quorum quenching by an N-acyl-homoserine lactone acylase from Pseudomonas aeruginosa PAO1
- PMID: 16495538
- PMCID: PMC1418629
- DOI: 10.1128/IAI.74.3.1673-1682.2006
Quorum quenching by an N-acyl-homoserine lactone acylase from Pseudomonas aeruginosa PAO1
Abstract
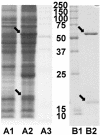
The virulence of the opportunistic human pathogen Pseudomonas aeruginosa PAO1 is controlled by an N-acyl-homoserine lactone (AHL)-dependent quorum-sensing system. During functional analysis of putative acylase genes in the P. aeruginosa PAO1 genome, the PA2385 gene was found to encode an acylase that removes the fatty acid side chain from the homoserine lactone (HSL) nucleus of AHL-dependent quorum-sensing signal molecules. Analysis showed that the posttranslational processing of the acylase and the hydrolysis reaction type are similar to those of the beta-lactam acylases, strongly suggesting that the PA2385 protein is a member of the N-terminal nucleophile hydrolase superfamily. In a bioassay, the purified acylase was shown to degrade AHLs with side chains ranging in length from 11 to 14 carbons at physiologically relevant low concentrations. The substituent at the 3' position of the side chain did not affect activity, indicating broad-range AHL quorum-quenching activity. Of the two main AHL signal molecules of P. aeruginosa PAO1, N-butanoyl-l-homoserine lactone (C4-HSL) and N-(3-oxododecanoyl)-l-homoserine lactone (3-oxo-C12-HSL), only 3-oxo-C12-HSL is degraded by the enzyme. Addition of the purified protein to P. aeruginosa PAO1 cultures completely inhibited accumulation of 3-oxo-C12-HSL and production of the signal molecule 2-heptyl-3-hydroxy-4(1H)-quinolone and reduced production of the virulence factors elastase and pyocyanin. Similar results were obtained when the PA2385 gene was overexpressed in P. aeruginosa. These results demonstrate that the protein has in situ quorum-quenching activity. The quorum-quenching AHL acylase may enable P. aeruginosa PAO1 to modulate its own quorum-sensing-dependent pathogenic potential and, moreover, offers possibilities for novel antipseudomonal therapies.
Figures




Similar articles
-
PA0305 of Pseudomonas aeruginosa is a quorum quenching acylhomoserine lactone acylase belonging to the Ntn hydrolase superfamily.Microbiology (Reading). 2011 Jul;157(Pt 7):2042-2055. doi: 10.1099/mic.0.043935-0. Epub 2011 Mar 3. Microbiology (Reading). 2011. PMID: 21372094
-
Identification of QuiP, the product of gene PA1032, as the second acyl-homoserine lactone acylase of Pseudomonas aeruginosa PAO1.Appl Environ Microbiol. 2006 Feb;72(2):1190-7. doi: 10.1128/AEM.72.2.1190-1197.2006. Appl Environ Microbiol. 2006. PMID: 16461666 Free PMC article.
-
Utilization of acyl-homoserine lactone quorum signals for growth by a soil pseudomonad and Pseudomonas aeruginosa PAO1.Appl Environ Microbiol. 2003 Oct;69(10):5941-9. doi: 10.1128/AEM.69.10.5941-5949.2003. Appl Environ Microbiol. 2003. PMID: 14532048 Free PMC article.
-
Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing.Annu Rev Genet. 2001;35:439-68. doi: 10.1146/annurev.genet.35.102401.090913. Annu Rev Genet. 2001. PMID: 11700290 Review.
-
Quorum sensing molecule N-(3-oxododecanoyl)-l-homoserine lactone: An all-rounder in mammalian cell modification.J Oral Biosci. 2020 Mar;62(1):16-29. doi: 10.1016/j.job.2020.01.001. Epub 2020 Jan 23. J Oral Biosci. 2020. PMID: 31982630 Review.
Cited by
-
Metabolism and function of phenazines in bacteria: impacts on the behavior of bacteria in the environment and biotechnological processes.Appl Microbiol Biotechnol. 2010 May;86(6):1659-70. doi: 10.1007/s00253-010-2509-3. Epub 2010 Mar 30. Appl Microbiol Biotechnol. 2010. PMID: 20352425 Free PMC article. Review.
-
Characterization of N-acylhomoserine lactone-degrading bacteria associated with the Zingiber officinale (ginger) rhizosphere: co-existence of quorum quenching and quorum sensing in Acinetobacter and Burkholderia.BMC Microbiol. 2011 Mar 8;11:51. doi: 10.1186/1471-2180-11-51. BMC Microbiol. 2011. PMID: 21385437 Free PMC article.
-
Immobilized Acylase PvdQ Reduces Pseudomonas aeruginosa Biofilm Formation on PDMS Silicone.Front Chem. 2020 Feb 5;8:54. doi: 10.3389/fchem.2020.00054. eCollection 2020. Front Chem. 2020. PMID: 32117880 Free PMC article.
-
Quorum-quenching acylase reduces the virulence of Pseudomonas aeruginosa in a Caenorhabditis elegans infection model.Antimicrob Agents Chemother. 2009 Nov;53(11):4891-7. doi: 10.1128/AAC.00380-09. Epub 2009 Aug 31. Antimicrob Agents Chemother. 2009. PMID: 19721066 Free PMC article.
-
The Pseudomonas aeruginosa Orphan Quorum Sensing Signal Receptor QscR Regulates Global Quorum Sensing Gene Expression by Activating a Single Linked Operon.mBio. 2018 Aug 28;9(4):e01274-18. doi: 10.1128/mBio.01274-18. mBio. 2018. PMID: 30154259 Free PMC article.
References
-
- Arevalo-Ferro, C., M. Hentzer, G. Reil, A. Gorg, S. Kjelleberg, M. Givskov, K. Riedel, and L. Eberl. 2003. Identification of quorum-sensing regulated proteins in the opportunistic pathogen Pseudomonas aeruginosa by proteomics. Environ. Microbiol. 5:1350-1369. - PubMed
-
- Brannigan, J. A., G. Dodson, H. J. Duggleby, P. C. Moody, J. L. Smith, D. R. Tomchick, and A. G. Murzin. 1995. A protein catalytic framework with an N-terminal nucleophile is capable of self-activation. Nature 378:416-419. - PubMed
-
- Bruggink, A., E. C. Roos, and E. de Vroom. 1998. Penicillin acylase in the industrial production of β-lactam antibiotics. Org. Process Res. Dev. 2:128-133.
-
- Chhabra, S. R., P. Stead, N. J. Bainton, G. P. C. Salmond, G. S. A. B. Stewart, P. Williams, and B. W. Bycroft. 1993. Autoregulation of carbapenem biosynthesis in Erwinia carotovora by analogs of N-(3-oxohexanoyl)-l-homoserine lactone. J. Antibiot. 46:441-454. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

