Role of tumor necrosis factor alpha (TNF-alpha) and interleukin-10 in the pathogenesis of severe murine monocytotropic ehrlichiosis: increased resistance of TNF receptor p55- and p75-deficient mice to fatal ehrlichial infection
- PMID: 16495559
- PMCID: PMC1418656
- DOI: 10.1128/IAI.74.3.1846-1856.2006
Role of tumor necrosis factor alpha (TNF-alpha) and interleukin-10 in the pathogenesis of severe murine monocytotropic ehrlichiosis: increased resistance of TNF receptor p55- and p75-deficient mice to fatal ehrlichial infection
Abstract
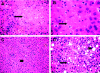
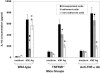
Intraperitoneal (i.p.) infection with a high dose of a highly virulent Ehrlichia strain (IOE) results in a toxic shock-like syndrome characterized by severe liver injury and systemic overproduction of tumor necrosis factor alpha (TNF-alpha) by CD8+ T cells. We examined the role of TNF-alpha and TNF receptors in high-dose-IOE-induced shock/liver injury. TNF receptor (TNFR) I/II-/- mice lacking both the p55 and p75 receptors for this cytokine were more resistant to IOE-induced liver injury than their wild-type background controls. TNFR I/II-/- mice survived longer, dying between 15 and 18 days, with evidence of mild liver necrosis/apoptosis. In contrast, wild-type mice were not rescued from the lethal effect of IOE by TNF-alpha neutralization. TNF-alpha-depleted mice developed severe liver injury and succumbed to disease between days 9 and 11 postinfection, similar to sham-treated, infected wild-type mice. Although IFN-gamma production in the spleens of IOE-infected TNFR I/II-/- and TNF-alpha-depleted mice was higher than that detected in wild-type controls, these mice had higher bacterial burdens than infected controls. Following high-dose IOE challenge, TNFR I/II-/- and TNF-alpha-depleted mice have an early increase in IL-10 levels in sera and spleens, which was produced mainly by adherent spleen cells. In contrast, a late burst of interleukin-10 (IL-10) was observed in control mice. Nonadherent spleen cells were the major source of IL-10 in IOE-infected wild-type mice. We conclude that TNFR I/II and TNF-alpha participate in Ehrlichia-induced shock and host defense by regulating liver injury and controlling ehrlichial burden. Our data suggest that fatal ehrlichiosis could be a multistep process, where TNF-alpha is not solely responsible for mortality.
Figures







References
-
- Bean, A. G., D. R. Roach, H. Briscoe, M. P. France, H. Korner, J. D. Sedgwick, and W. J. Britton. 1999. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J. Immunol. 162:3504-3511. - PubMed
-
- Bitsaktsis, C., J. Huntington, and G. Winslow. 2004. Production of IFN-gamma by CD4 T cells is essential for resolving ehrlichia infection. J. Immunol. 172:6894-6901. - PubMed
-
- Bogdan, C., M. Rollinghoff, and A. Diefenbach. 2002. The role of nitric oxide in innate immunity. Immunol. Rev. 173:17-26. - PubMed
-
- Chan, K. F., M. R. Siegel, and J. M. Lenardo. 2000. Signaling by the TNF receptor superfamily and T cell homeostasis. Immunity 13:419-422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

