Toll-like receptor 2 mediates alveolar macrophage response to Pneumocystis murina
- PMID: 16495560
- PMCID: PMC1418649
- DOI: 10.1128/IAI.74.3.1857-1864.2006
Toll-like receptor 2 mediates alveolar macrophage response to Pneumocystis murina
Abstract
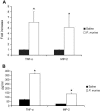
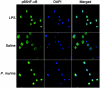
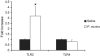
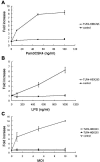
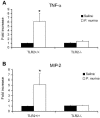
The innate immune response to Pneumocystis infection is not well understood. In this study, normal C57BL/6 mouse alveolar macrophages were found to respond to Pneumocystis murina organisms through Toll-like receptor 2 (TLR2), leading to the nuclear translocation of NF-kappaB and the production of proinflammatory cytokine tumor necrosis factor alpha (TNF-alpha) and chemokine macrophage inflammatory protein 2 (MIP-2). P. murina stimulation of normal alveolar macrophages from C57BL/6 mice resulted in increased TLR2 transcription but not increased TLR4 transcription. In gain-of-function studies with HEK293 cells expressing TLR2 or TLR4, only TLR2 was found to stimulate an NF-kappaB response to P. murina. TNF-alpha and MIP-2 production in response to P. murina by mouse alveolar macrophages was inhibited by a monoclonal antibody that specifically blocked the ligand-binding ability of TLR2. Alveolar macrophages from TLR2 knockout (TLR2-/-) mice showed little increase in TNF-alpha and MIP-2 mRNA levels upon P. murina stimulation. An in vivo study showed that TLR2-/- mice challenged with P. murina had reduced cytokine responses. These results indicate that TLR2 plays a major role in the innate immune response to P. murina.
Figures
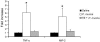
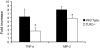
Similar articles
-
Toll-like receptor 2 mediates inflammatory cytokine induction but not sensitization for liver injury by Propioni- bacterium acnes.J Leukoc Biol. 2005 Dec;78(6):1255-64. doi: 10.1189/jlb.0804448. Epub 2005 Oct 4. J Leukoc Biol. 2005. PMID: 16204620
-
Pneumocystis-mediated IL-8 release by macrophages requires coexpression of mannose receptors and TLR2.J Leukoc Biol. 2007 Jan;81(1):205-11. doi: 10.1189/jlb.1005580. Epub 2006 Oct 4. J Leukoc Biol. 2007. PMID: 17020928
-
Negative regulatory role of mannose receptors on human alveolar macrophage proinflammatory cytokine release in vitro.J Leukoc Biol. 2005 Sep;78(3):665-74. doi: 10.1189/jlb.1204699. Epub 2005 Jul 6. J Leukoc Biol. 2005. PMID: 16000387
-
Role of CD44 in Regulating TLR2 Activation of Human Macrophages and Downstream Expression of Proinflammatory Cytokines.J Immunol. 2018 Jan 15;200(2):758-767. doi: 10.4049/jimmunol.1700713. Epub 2017 Dec 1. J Immunol. 2018. PMID: 29196459 Free PMC article.
-
Review: Chemical and structural modifications of pulmonary collectins and their functional consequences.Innate Immun. 2010 Jun;16(3):175-82. doi: 10.1177/1753425910368871. Epub 2010 Apr 27. Innate Immun. 2010. PMID: 20423921 Free PMC article. Review.
Cited by
-
Current understanding of Pneumocystis immunology.Future Microbiol. 2010 Jan;5(1):43-65. doi: 10.2217/fmb.09.116. Future Microbiol. 2010. PMID: 20020829 Free PMC article. Review.
-
Clearance of Pneumocystis murina infection is not dependent on MyD88.Microbes Infect. 2014 Jun;16(6):522-7. doi: 10.1016/j.micinf.2014.03.005. Epub 2014 Mar 25. Microbes Infect. 2014. PMID: 24680862 Free PMC article.
-
Scavenger receptor A dampens induction of inflammation in response to the fungal pathogen Pneumocystis carinii.Infect Immun. 2007 Aug;75(8):3999-4005. doi: 10.1128/IAI.00393-07. Epub 2007 Jun 4. Infect Immun. 2007. PMID: 17548480 Free PMC article.
-
The Interaction of Pneumocystis with the C-Type Lectin Receptor Mincle Exerts a Significant Role in Host Defense against Infection.J Immunol. 2017 May 1;198(9):3515-3525. doi: 10.4049/jimmunol.1600744. Epub 2017 Mar 15. J Immunol. 2017. PMID: 28298521 Free PMC article.
-
Vitamin D as Supplemental Therapy for Pneumocystis Pneumonia.Antimicrob Agents Chemother. 2015 Dec 14;60(3):1289-97. doi: 10.1128/AAC.02607-15. Antimicrob Agents Chemother. 2015. PMID: 26666941 Free PMC article.
References
-
- Baker-LePain, J. C., M. Sarzotti, and C. V. Nicchitta. 2004. Glucose-regulated protein 94/glycoprotein 96 elicits bystander activation of CD4+ T cell Th1 cytokine production in vivo. J. Immunol. 172:4195-4203. - PubMed
-
- Bartlett, M. S., S. F. Queener, M. M. Durkin, M. A. Shaw, and J. W. Smith. 1992. Inoculated mouse model of Pneumocystis carinii infection. Diagn. Microbiol. Infect. Dis. 15:129-134. - PubMed
-
- Beck, J. M., R. L. Newbury, B. E. Palmer, M. L. Warnock, P. K. Byrd, and H. B. Kaltreider. 1996. Role of CD8+ lymphocytes in host defense against Pneumocystis carinii in mice. J. Lab. Clin. Med. 128:477-487. - PubMed
-
- Benfield, T. L., J. Vestbo, J. Junge, T. L. Nielsen, A. B. Jensen, and J. D. Lundgren. 1995. Prognostic value of interleukin-8 in AIDS-associated Pneumocystis carinii pneumonia. Am. J. Respir. Crit. Care Med. 151:1058-1062. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

