Two monoclonal antibodies with defined epitopes of P44 major surface proteins neutralize Anaplasma phagocytophilum by distinct mechanisms
- PMID: 16495562
- PMCID: PMC1418626
- DOI: 10.1128/IAI.74.3.1873-1882.2006
Two monoclonal antibodies with defined epitopes of P44 major surface proteins neutralize Anaplasma phagocytophilum by distinct mechanisms
Abstract
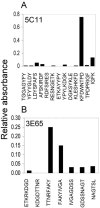
Anaplasma phagocytophilum is an obligatory intracellular bacterium that causes human granulocytic anaplasmosis. The polymorphic 44-kDa major outer membrane proteins of A. phagocytophilum are dominant antigens recognized by patients and infected animals. However, the ability of anti-P44 antibody to neutralize the infection has been unclear due to a mixture of P44 proteins with diverse hypervariable region amino acid sequences expressed by a given bacterial population and lack of epitope-defined antibodies. Monoclonal antibodies (MAbs) 5C11 and 3E65 are directed to different domains of P44 proteins, the N-terminal conserved region and P44-18 central hypervariable region, respectively. Passive immunization with either MAb 5C11 or 3E65 partially protects mice from infection with A. phagocytophilum. In the present study, we demonstrated that the two monoclonal antibodies recognize bacterial surface-exposed epitopes of naturally folded P44 proteins and mapped these epitopes to specific peptide sequences. The two MAbs almost completely blocked the infection of the A. phagocytophilum population that predominantly expressed P44-18 in HL-60 cells by distinct mechanisms: MAb 5C11 blocked the binding, but MAb 3E65 did not block binding or internalization. Instead, MAb 3E65 inhibited internalized A. phagocytophilum to develop into microcolonies called morulae. Some plasma from experimentally infected horses and mice reacted with these two epitopes. Taken together, these data indicate the presence of at least two distinct bacterial surface-exposed neutralization epitopes in P44 proteins. The results indicate that antibodies directed to certain epitopes of P44 proteins have a critical role in inhibiting A. phagocytophilum infection of host cells.
Figures

Similar articles
-
Anaplasma phagocytophilum has a functional msp2 gene that is distinct from p44.Infect Immun. 2004 Jul;72(7):3883-9. doi: 10.1128/IAI.72.7.3883-3889.2004. Infect Immun. 2004. PMID: 15213131 Free PMC article.
-
Insight of diagnostic performance using B-cell epitope antigens derived from triple P44-related proteins of Anaplasma phagocytophilum.Diagn Microbiol Infect Dis. 2019 Oct;95(2):125-130. doi: 10.1016/j.diagmicrobio.2019.05.008. Epub 2019 May 18. Diagn Microbiol Infect Dis. 2019. PMID: 31182246
-
Porin activity of Anaplasma phagocytophilum outer membrane fraction and purified P44.J Bacteriol. 2007 Mar;189(5):1998-2006. doi: 10.1128/JB.01548-06. Epub 2006 Dec 15. J Bacteriol. 2007. PMID: 17172334 Free PMC article.
-
Persistent Infections and Immunity in Ruminants to Arthropod-Borne Bacteria in the Family Anaplasmataceae.Annu Rev Anim Biosci. 2016;4:177-97. doi: 10.1146/annurev-animal-022513-114206. Epub 2015 Dec 23. Annu Rev Anim Biosci. 2016. PMID: 26734888 Review.
-
Type IV secretion in the obligatory intracellular bacterium Anaplasma phagocytophilum.Cell Microbiol. 2010 Sep 1;12(9):1213-21. doi: 10.1111/j.1462-5822.2010.01500.x. Epub 2010 Jul 28. Cell Microbiol. 2010. PMID: 20670295 Free PMC article. Review.
Cited by
-
Mechanisms of obligatory intracellular infection with Anaplasma phagocytophilum.Clin Microbiol Rev. 2011 Jul;24(3):469-89. doi: 10.1128/CMR.00064-10. Clin Microbiol Rev. 2011. PMID: 21734244 Free PMC article. Review.
-
Anaplasma phagocytophilum MSP2(P44)-18 predominates and is modified into multiple isoforms in human myeloid cells.Infect Immun. 2008 May;76(5):2090-8. doi: 10.1128/IAI.01594-07. Epub 2008 Feb 19. Infect Immun. 2008. PMID: 18285495 Free PMC article.
-
The Anaplasma phagocytophilum PleC histidine kinase and PleD diguanylate cyclase two-component system and role of cyclic Di-GMP in host cell infection.J Bacteriol. 2009 Feb;191(3):693-700. doi: 10.1128/JB.01218-08. Epub 2008 Oct 31. J Bacteriol. 2009. PMID: 18978058 Free PMC article.
-
Differential expression and glycosylation of anaplasma phagocytophilum major surface protein 2 paralogs during cultivation in sialyl Lewis x-deficient host cells.Infect Immun. 2009 May;77(5):1746-56. doi: 10.1128/IAI.01530-08. Epub 2009 Feb 17. Infect Immun. 2009. PMID: 19223475 Free PMC article.
-
Anaplasma phagocytophilum and Ehrlichia chaffeensis: subversive manipulators of host cells.Nat Rev Microbiol. 2010 May;8(5):328-39. doi: 10.1038/nrmicro2318. Epub 2010 Apr 7. Nat Rev Microbiol. 2010. PMID: 20372158 Review.
References
-
- Abbott, J. R., G. H. Palmer, C. J. Howard, J. C. Hope, and W. C. Brown. 2004. Anaplasma marginale major surface protein 2 CD4+-T-cell epitopes are evenly distributed in conserved and hypervariable regions (HVR), whereas linear B-cell epitopes are predominantly located in the HVR. Infect. Immun. 72:7360-7366. - PMC - PubMed
-
- Bakken, J. S., J. S. Dumler, S. M. Chen, M. R. Eckman, L. L. V. Etta, and D. H. Walker. 1994. Human granulocytic ehrlichiosis in the upper Midwest United States. A new species emerging? JAMA 272:212-218. - PubMed
-
- Barbet, A. F., P. F. M. Meeus, M. Belanger, M. V. Bowie, J. Yi, A. M. Lundgren, A. R. Alleman, S. J. Wong, F. K. Chu, U. G. Munderloh, and S. D. Jauron. 2003. Expression of multiple outer membrane protein sequence variants from a single genomic locus of Anaplasma phagocytophilum. Infect. Immun. 71:1706-1718. - PMC - PubMed
-
- Barbour, A. G. 1990. Antigenic variation of a relapsing fever Borrelia species. Annu. Rev. Microbiol. 44:155-171. - PubMed
-
- Blouin, E. F., J. T. Saliki, J. de la Fuente, J. C. Garcia-Garcia, and K. M. Kocan. 2003. Antibodies to Anaplasma marginale major surface proteins 1a and 1b inhibit infectivity for cultured tick cells. Vet. Parasitol. 111:247-260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

