Amelioration of influenza-induced pathology in mice by coinfection with Trichinella spiralis
- PMID: 16495568
- PMCID: PMC1418664
- DOI: 10.1128/IAI.74.3.1924-1932.2006
Amelioration of influenza-induced pathology in mice by coinfection with Trichinella spiralis
Abstract
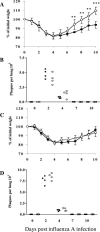
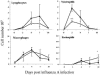
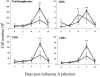
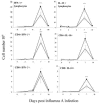
Illness due to respiratory virus infection is often induced by excessive infiltration of cells into pulmonary tissues, leading to airway occlusion. We show here that infection with Trichinella spiralis results in lower levels of tumor necrosis factor in bronchoalveolar lavage fluid and inhibits cellular recruitment into the airways of mice coinfected with influenza A virus. Infiltration of neutrophils and CD4+ and CD8+ lymphocytes was reduced, resulting in animals gaining weight more rapidly following the initial phase of infection. Influenza resulted in a generalized increase in vascular permeability in pulmonary tissues, and this was suppressed by parasite infection, although the effects were restricted to the early phase of trichinosis. Moreover, the number of cells producing interleukin-10 (IL-10), and the local levels of this cytokine, were reduced, suggesting that amelioration of pulmonary pathology by parasite infection occurs independently of IL-10 production.
Figures





References
-
- Bany, J., M. K. Janiak, and W. Budzynski. 1992. Activity of natural killer (NK) cells in the course of experimental trichinellosis in mice. Wiad. Parazytol. 38:117-126. - PubMed
-
- Biron, C. A., and L. Brossay. 2001. NK cells and NKT cells in innate defense against viral infections. Curr. Opin. Immunol. 13:458-464. - PubMed
-
- Bliss, S. K., A. Alcaraz, and J. A. Appleton. 2003. IL-10 prevents liver necrosis during murine infection with Trichinella spiralis. J. Immunol. 171:3142-3147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

