Selective binding of Borrelia burgdorferi OspE paralogs to factor H and serum proteins from diverse animals: possible expansion of the role of OspE in Lyme disease pathogenesis
- PMID: 16495576
- PMCID: PMC1418677
- DOI: 10.1128/IAI.74.3.1967-1972.2006
Selective binding of Borrelia burgdorferi OspE paralogs to factor H and serum proteins from diverse animals: possible expansion of the role of OspE in Lyme disease pathogenesis
Abstract
The binding of Borrelia burgdorferi OspE, OspF, and family 163 (Elp) proteins to factor H/factor H-like protein 1 (FHL-1) and other serum proteins from different animals was assessed. OspE paralogs bound factor H and unidentified serum proteins from a subset of animals, while OspF and Elp proteins did not. These data advance our understanding of factor H binding, the host range of the Lyme spirochetes, and the expanding role of OspE in pathogenesis.
Figures
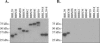
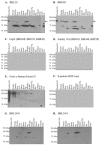
References
-
- Alitalo, A., T. Meri, T. Chen, H. Lankinen, Z.-Z. Cheng, T. S. Jokiranta, I. J. T. Seppala, P. Lahdenne, P. S. Hefty, D. R. Akins, and S. Meri. 2004. Lysine-dependent multipoint binding of the Borrelia burgdorferi virulence factor outer surface protein E to the C terminus of factor H. J. Immunol. 172:6195-6201. - PubMed
-
- Alitalo, A., T. Meri, H. Lankinen, I. Seppala, P. Lahdenne, P. S. Hefty, D. Akins, and S. Meri. 2002. Complement inhibitor factor H binding to Lyme disease spirochetes is mediated by inducible expression of multiple plasmid-encoded outer surface protein E paralogs. J. Immunol. 169:3847-3853. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

