Survival benefit associated with human anti-mouse antibody (HAMA) in patients with B-cell malignancies
- PMID: 16496145
- PMCID: PMC11030743
- DOI: 10.1007/s00262-006-0148-4
Survival benefit associated with human anti-mouse antibody (HAMA) in patients with B-cell malignancies
Abstract
Background: About one-third of patients with relapsed B-cell malignancies develop human anti-mouse antibody (HAMA) following mouse antibody treatment. The purpose of this study was to assess the relationship between HAMA and survival in patients given a mouse anti-lymphoma monoclonal antibody (mAb), Lym-1, directed against a unique epitope of HLA-DR antigen that is up-regulated on malignant B-cells.
Methods: ELISA was used to quantify HAMA in 51 patients with B-cell malignancies treated with iodine-131 (131I) labeled Lym-1. Sera were collected prior to and following radioimmunotherapy (RIT) with 131I-Lym-1 until documented to be HAMA negative or throughout lifetime. Univariate, then multivariate analyses including other risk factors, were used to analyze the relationship of HAMA to survival. The relationships of HAMA to prior chemotherapies and to absolute lymphocyte counts prior to RIT were also assessed.
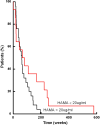
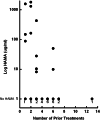
Results: Eighteen of 51 patients (35%) developed HAMA following RIT (range of ultimate maximum titers, 6.6-1,802 microg/ml). Using the time dependent Cox proportional hazards model, maximum HAMA titers were associated with survival (P=0.02). HAMA continued to be significant for survival in multivariate analyses that included known risk factors. In Landmark analysis of 39 patients that survived at least 16 weeks, median survival of patients with HAMA less than 5 microg/ml was 61 versus 103 weeks for patients with HAMA equal or greater than 5 microg/ml at 16 weeks (P=0.02). The median survival of the five patients with highest maximum HAMA titers was 244 weeks. At 16 weeks, there was an inverse correlation between the maximum HAMA titer and the number of previous chemotherapies (P<0.003). Absolute lymphocyte counts prior to 131I-Lym-1 treatment for patients that seroconverted were higher than those for patients that did not seroconvert (P=0.01).
Conclusions: Patients with B-cell malignancies that developed high HAMA titers had longer survival that was not explained by risk factors or histologic grade, suggesting the importance of the immune system.
Figures
Similar articles
-
Prolonged survival associated with immune response in a patient treated with Lym-1 mouse monoclonal antibody.Cancer Biother Radiopharm. 1998 Feb;13(1):1-12. doi: 10.1089/cbr.1998.13.1. Cancer Biother Radiopharm. 1998. PMID: 10850337
-
Analysis of antiglobulin (HAMA) response in a group of patients with B-lymphocytic malignancies treated with 131I-Lym-1.Int J Biol Markers. 1995 Apr-Jun;10(2):67-74. doi: 10.1177/172460089501000201. Int J Biol Markers. 1995. PMID: 7561241 Clinical Trial.
-
Treatment-related parameters predicting efficacy of Lym-1 radioimmunotherapy in patients with B-lymphocytic malignancies.Clin Cancer Res. 1997 Aug;3(8):1253-60. Clin Cancer Res. 1997. PMID: 9815807 Clinical Trial.
-
Milestones in the development of Lym-1 therapy.Hybridoma. 1999 Feb;18(1):1-11. doi: 10.1089/hyb.1999.18.1. Hybridoma. 1999. PMID: 10211782 Review.
-
Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia.Drugs. 2003;63(8):803-43. doi: 10.2165/00003495-200363080-00005. Drugs. 2003. PMID: 12662126 Review.
Cited by
-
Therapeutic radionuclides in nuclear medicine: current and future prospects.J Zhejiang Univ Sci B. 2014 Oct;15(10):845-63. doi: 10.1631/jzus.B1400131. J Zhejiang Univ Sci B. 2014. PMID: 25294374 Free PMC article. Review.
-
False elevation of human chorionic gonadotropin in a patient with testicular cancer.Nat Rev Urol. 2010 Apr;7(4):230-3. doi: 10.1038/nrurol.2010.10. Nat Rev Urol. 2010. PMID: 20383188
-
Immune cell engagers in solid tumors: promises and challenges of the next generation immunotherapy.ESMO Open. 2021 Feb;6(1):100046. doi: 10.1016/j.esmoop.2020.100046. Epub 2021 Jan 25. ESMO Open. 2021. PMID: 33508733 Free PMC article. Review.
-
Immunogenicity of Iodine 131 chimeric tumor necrosis therapy monoclonal antibody in advanced lung cancer patients.Cancer Immunol Immunother. 2008 May;57(5):677-84. doi: 10.1007/s00262-007-0406-0. Epub 2007 Oct 13. Cancer Immunol Immunother. 2008. PMID: 17934732 Free PMC article. Clinical Trial.
-
Novel monoclonal antibodies for cancer treatment: the trifunctional antibody catumaxomab (removab).J Cancer. 2011;2:309-16. doi: 10.7150/jca.2.309. Epub 2011 May 25. J Cancer. 2011. PMID: 21716847 Free PMC article.
References
-
- DeNardo GL, DeNardo SJ, Goldstein DS, Kroger LA, Lamborn KR, Levy NB, McGahan JP, Salako QA, Shen S, Lewis JP. Maximum tolerated dose, toxicity, and efficacy of 131I-Lym-1 antibody for fractionated radioimmunotherapy of non-Hodgkin’s lymphoma. J Clin Oncol. 1998;16:3246–3256. - PubMed
-
- DeNardo GL, DeNardo SJ, Lamborn KR, Goldstein DS, Levy NB, Lewis JP, O’Grady LF, Raventos A, Kroger LA, Macey DJ, McGahan JP, Mills SL, Shen S. Low-dose fractionated radioimmunotherapy for B-cell malignancies using 131I-Lym-1 antibody. Cancer Biother Radiopharm. 1998;13:239–254. - PubMed
-
- Kaminski MS, Zelenetz AD, Press OW, Saleh M, Leonard J, Fehrenbacher L, Lister TA, Stagg RJ, Tidmarsh GF, Kroll S, Wahl RL, Knox SJ, Vose JM. Pivotal study of iodine I 131 tositumomab for chemotherapy-refractory low-grade or transformed low-grade B-cell non-Hodgkin’s lymphomas. J Clin Oncol. 2001;19:3918–3928. - PubMed
-
- McLaughlin P, Grillo-Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, Heyman MR, Bence-Bruckler I, White CA, Cabanillas F, Jain V, Ho AD, Lister J, Wey K, Shen D, Dallaire BK. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–2833. - PubMed
-
- Witzig TE, Gordon LI, Cabanillas F, Czuczman MS, Emmanouilides C, Joyce R, Pohlman BL, Bartlett NL, Wiseman GA, Padre N, Grillo-Lopez AJ, Multani P, White CA. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed refractory low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphoma. J Clin Oncol. 2002;20:2453–2463. doi: 10.1200/JCO.2002.11.076. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

