Studies on a total synthesis of the microbial immunosuppresive agent FR901483
- PMID: 16496992
- PMCID: PMC2527037
- DOI: 10.1021/jo052466b
Studies on a total synthesis of the microbial immunosuppresive agent FR901483
Abstract
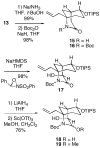
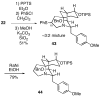
A strategy is outlined for construction of the fungal immunosuppressant FR901483 (1). It was possible to convert 1,4-cyclohexanedione monoethylene ketal in five simple steps to iodoacetamide ketone 10, which was cyclized in good yield to the key bridged keto lactam 11 containing the A/B 2-azabicyclo[3.3.1]nonane ring system of the natural product. This intermediate could be transformed to N-Boc lactam 16, whose derived enolate underwent stereoselective hydroxylation with the Davis oxaziridine to produce alcohol 17 having the desired C-2 configuration. Compound 17 was then converted in three steps to alkoxy carbamate 20. The N-acyliminium ion derived from intermediate 20 could be alkylated in good overall yield with p-methoxybenzylmagnesium chloride to afford a 5:4 mixture of the desired PMB product 21 and the epimer 23. In an attempt to improve the stereoselectivity in this alkylation, the inverted C-4 protected alcohol N-Boc lactam 33 was prepared and its enolate was hydroxylated. Inexplicably, the product of this reaction was the undesired equatorial alcohol 34. Some model systems were investigated toward annulation of the C-ring of the natural product. It was found that homoallylic amine 40 could be cyclized with PhSCl in the presence of silica gel to generate the desired 5-endo tetracyclic product 42 in moderate yield. This cyclization protocol was also successfully applied to the actual FR901483 system 22, leading to the requisite tricycle 43.
Figures
References
-
- Sakamoto K, Tsujii E, Abe F, Nakanishi T, Yamashita M, Shigematsu N, Izumi S, Okuhara M. J Antibiot. 1996;49:37. - PubMed
-
-
For total syntheses of FR901483 see: Snider BB, Lin H. J Am Chem Soc. 1999;121:7778.Scheffler G, Seike H, Sorensen EJ. Angew Chem Int Ed Engl. 2000;39:4593.Ousmer M, Braun NA, Ciufolini MA. Org Lett. 2001;3:765.Maeng JH, Funk RL. Org Lett. 2001;3:1125.Ousmer M, Braun NA, Bavoux C, Perrin M, Ciufolini MA. J Am Chem Soc. 2001;123:7534.Kan T, Fujimoto T, Ieda S, Asoh Y, Kitaoka H, Fukuyama T. Org Lett. 2004;6:2729.For a formal total synthesis see: Brummond KM, Hong S. J Org Chem. 2005;70:907.
-
-
-
For other synthetic approaches to this metabolite and some analogs see: Snider BB, Lin H, Foxman BM. J Org Chem. 1998;63:6442.Bonjoch J, Diaba F, Puigbo G, Sole D, Segarra V, Santamaria L, Beleta J, Ryder H, Palacios JM. Bioorg Med Chem. 1999;7:2891.Suzuki H, Yamazaki N, Kibayashi C. Tetrahedron Lett. 2001;42:3013.Brummond KM, Lu J. Org Lett. 2001;3:1347.Wardrop DJ, Zhang W. Org Lett. 2001;3:2353.Bonjoch J, Diaba F, Puigbo G, Peidro E, Sole D. Tetrahedron Lett. 2003;44:8387.Panchaud P, Ollivier C, Renaud P, Zigmantas S. J Org Chem. 2004;69:2755.
-
-
-
Taken in part from the Ph.D. thesis of J.E. Kropf, The Pennsylvania State University, 2002.
-
-
-
See for example: Marvell EN, Sturmer D, Rowell C. Tetrahedron. 1966;22:861.Lednicer D. J Med Chem. 1977;20:171.Boger DL, Patel M, Mullican MD. Tetrahedron Lett. 1982;23:4559.Quirante J, Escolano C, Merino A, Bonjoch J. J Org Chem. 1998;63:968.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous