Morphological analysis of tumor cell/endothelial cell interactions under shear flow
- PMID: 16497312
- PMCID: PMC1961634
- DOI: 10.1016/j.jbiomech.2006.01.001
Morphological analysis of tumor cell/endothelial cell interactions under shear flow
Abstract
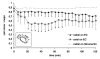
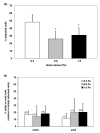
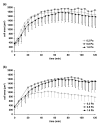
In the process of hematogenous cancer metastasis, tumor cells (TCs) must shed into the blood stream, survive in the blood circulation, migrate through the vascular endothelium (extravasation) and proliferate in the target organs. However, the precise mechanisms by which TCs penetrate the endothelial cell (EC) junctions remain one of the least understood aspects of TC extravasation. This question has generally been addressed under static conditions, despite the important role of flow induced mechanical stress on the circulating cell-endothelium interactions. Moreover, flow studies were generally focused on transient or firm adhesion steps of TC-EC interactions and did not consider TCs spreading or extravasation. In this paper, we used a parallel-plate flow chamber to investigate TC-EC interactions under flow conditions. An EC monolayer was cultured on the lower plate of the flow chamber to model the endothelial barrier. Circulating TCs were introduced into the flow channel under a well-defined flow field and TC cell shape changes on the EC monolayer were followed in vitro with live phase contrast and fluorescence microscopy. Two spreading patterns were observed: radial spreading which corresponds to TC extravasation, and axial spreading where TCs formed a mosaic TC-EC monolayer. By investigating the changes in area and minor/major aspect ratio, we have established a simple quantitative basis for comparing spreading modes under various shear stresses. Contrary to radial spreading, the extent of axial spreading was increased by shear stress.
Figures





Similar articles
-
Tumor cell/endothelial cell tight contact upregulates endothelial adhesion molecule expression mediated by NFkappaB: differential role of the shear stress.Exp Cell Res. 2010 Feb 15;316(4):615-26. doi: 10.1016/j.yexcr.2009.11.015. Epub 2009 Nov 26. Exp Cell Res. 2010. PMID: 19944683
-
Flow cytometric assay for quantitative and qualitative evaluation of adhesive interactions of tumor cells with endothelial cells.Microvasc Res. 2008 Aug;76(2):134-8. doi: 10.1016/j.mvr.2008.03.004. Epub 2008 Apr 1. Microvasc Res. 2008. PMID: 18675997
-
Vascular endothelial wound closure under shear stress: role of membrane fluidity and flow-sensitive ion channels.J Appl Physiol (1985). 2005 Jun;98(6):2355-62. doi: 10.1152/japplphysiol.01136.2004. Epub 2005 Feb 10. J Appl Physiol (1985). 2005. PMID: 15705727
-
Glycomechanics of the metastatic cascade: tumor cell-endothelial cell interactions in the circulation.Ann Biomed Eng. 2012 Apr;40(4):790-805. doi: 10.1007/s10439-011-0463-6. Epub 2011 Nov 19. Ann Biomed Eng. 2012. PMID: 22101756 Review.
-
Role of tumor cell adhesion and migration in organ-specific metastasis formation.Onkologie. 2004 Dec;27(6):577-82. doi: 10.1159/000081343. Onkologie. 2004. PMID: 15591720 Review.
Cited by
-
Review: Mechanotransduction in ovarian cancer: Shearing into the unknown.APL Bioeng. 2018 Jun 7;2(3):031701. doi: 10.1063/1.5024386. eCollection 2018 Sep. APL Bioeng. 2018. PMID: 31069311 Free PMC article. Review.
-
Modelling how curved active proteins and shear flow pattern cellular shape and motility.Front Cell Dev Biol. 2023 May 31;11:1193793. doi: 10.3389/fcell.2023.1193793. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37325558 Free PMC article.
-
Viscoelastic Properties in Cancer: From Cells to Spheroids.Cells. 2021 Jul 6;10(7):1704. doi: 10.3390/cells10071704. Cells. 2021. PMID: 34359874 Free PMC article.
-
Unraveling the Receptor-Ligand Interactions between Bladder Cancer Cells and the Endothelium Using AFM.Biophys J. 2017 Mar 28;112(6):1246-1257. doi: 10.1016/j.bpj.2017.01.033. Biophys J. 2017. PMID: 28355551 Free PMC article.
-
Mechanotransduction in the endothelium: role of membrane proteins and reactive oxygen species in sensing, transduction, and transmission of the signal with altered blood flow.Antioxid Redox Signal. 2014 Feb 20;20(6):899-913. doi: 10.1089/ars.2013.5624. Epub 2014 Jan 22. Antioxid Redox Signal. 2014. PMID: 24328670 Free PMC article. Review.
References
-
- Al-Mehdi AB, Tozawa K, Fisher AB, Shientag L, Lee A, Muschel RJ. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat Med. 2000;6:100–102. - PubMed
-
- Burdick MM, McCaffery JM, Kim YS, Bochner BS, Konstantopoulos K. Colon carcinoma cell glycolipids, integrins, and other glycoproteins mediate adhesion to HUVECs under flow. Am J Physiol Cell Physiol. 2003;284:977–987. - PubMed
-
- Burns AR, Walker DC, Brown ES, Thurmon LT, Bowden RA, Keese CR, Simon SI, Entman ML, Smith CW. Neutrophil transendothelial migration is independent of tight junctions and occurs preferentially at tricellular corners. J Immunol. 1997;159:2893–2903. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

