Expression cloning of three Rhizobium leguminosarum lipopolysaccharide core galacturonosyltransferases
- PMID: 16497674
- PMCID: PMC2814240
- DOI: 10.1074/jbc.M513864200
Expression cloning of three Rhizobium leguminosarum lipopolysaccharide core galacturonosyltransferases
Abstract
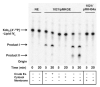
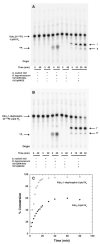
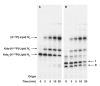
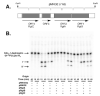
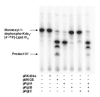
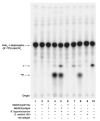
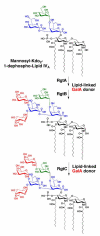
The lipid A and core regions of the lipopolysaccharide in Rhizobium leguminosarum, a nitrogen-fixing plant endosymbiont, are strikingly different from those of Escherichia coli. In R. leguminosarum lipopolysaccharide, the inner core is modified with three galacturonic acid (GalA) moieties, two on the distal 3-deoxy-D-manno-octulosonic acid (Kdo) unit and one on the mannose residue. Here we describe the expression cloning of three novel GalA transferases from a 22-kb R. leguminosarum genomic DNA insert-containing cosmid (pSGAT). Two of these enzymes modify the substrate, Kdo2-[4'-(32)P]lipid IV(A) and its 1-dephosphorylated derivative on the distal Kdo residue, as indicated by mild acid hydrolysis. The third enzyme modifies the mannose unit of the substrate mannosyl-Kdo2-1-dephospho-[4'-(32)P]lipid IV(A). Sequencing of a 7-kb subclone derived from pSGAT revealed three putative membrane-bound glycosyltransferases, now designated RgtA, RgtB, and RgtC. Transfer by tri-parental mating of these genes into Sinorhizobium meliloti 1021, a strain that lacks these particular GalA residues, results in the heterologous expression of the GalA transferase activities seen in membranes of cells expressing pSGAT. Reconstitution experiments with the individual genes demonstrated that the activity of RgtA precedes and is necessary for the subsequent activity of RgtB, which is followed by the activity of RgtC. Electrospray ionization-tandem mass spectrometry and gas-liquid chromatography of the product generated in vitro by RgtA confirmed the presence of a GalA moiety. No in vitro activity was detected when RgtA was expressed in Escherichia coli unless Rhizobiaceae membranes were also included.
Figures





Similar articles
-
Dodecaprenyl phosphate-galacturonic acid as a donor substrate for lipopolysaccharide core glycosylation in Rhizobium leguminosarum.J Biol Chem. 2006 May 5;281(18):12879-87. doi: 10.1074/jbc.M513865200. Epub 2006 Feb 23. J Biol Chem. 2006. PMID: 16497671 Free PMC article.
-
Cloning and overexpression of glycosyltransferases that generate the lipopolysaccharide core of Rhizobium leguminosarum.J Biol Chem. 1998 Oct 9;273(41):26432-40. doi: 10.1074/jbc.273.41.26432. J Biol Chem. 1998. PMID: 9756877
-
Characterization of galacturonosyl transferase genes rgtA, rgtB, rgtC, rgtD, and rgtE responsible for lipopolysaccharide synthesis in nitrogen-fixing endosymbiont Rhizobium leguminosarum: lipopolysaccharide core and lipid galacturonosyl residues confer membrane stability.J Biol Chem. 2012 Jan 6;287(2):935-49. doi: 10.1074/jbc.M111.311571. Epub 2011 Nov 22. J Biol Chem. 2012. PMID: 22110131 Free PMC article.
-
Expression cloning and characterization of the C28 acyltransferase of lipid A biosynthesis in Rhizobium leguminosarum.J Biol Chem. 2002 Aug 9;277(32):28959-71. doi: 10.1074/jbc.M204525200. Epub 2002 May 17. J Biol Chem. 2002. PMID: 12019272 Free PMC article.
-
Lipopolysaccharide core glycosylation in Rhizobium leguminosarum. An unusual mannosyl transferase resembling the heptosyl transferase I of Escherichia coli.J Biol Chem. 1996 Dec 13;271(50):32119-25. J Biol Chem. 1996. PMID: 8943265
Cited by
-
Escherichia coli mutants that synthesize dephosphorylated lipid A molecules.Biochemistry. 2010 Sep 28;49(38):8325-37. doi: 10.1021/bi101253s. Biochemistry. 2010. PMID: 20795687 Free PMC article.
-
Roles of predicted glycosyltransferases in the biosynthesis of the Rhizobium etli CE3 O antigen.J Bacteriol. 2013 May;195(9):1949-58. doi: 10.1128/JB.02080-12. Epub 2013 Feb 22. J Bacteriol. 2013. PMID: 23435981 Free PMC article.
-
The calcium-stimulated lipid A 3-O deacylase from Rhizobium etli is not essential for plant nodulation.Biochim Biophys Acta. 2012 Jul;1831(7):1250-9. doi: 10.1016/j.bbalip.2013.04.002. Epub 2013 Apr 12. Biochim Biophys Acta. 2012. PMID: 23583844 Free PMC article.
-
Synthesis of N-acetyl-d-quinovosamine in Rhizobium etli CE3 is completed after its 4-keto-precursor is linked to a carrier lipid.Microbiology (Reading). 2017 Dec;163(12):1890-1901. doi: 10.1099/mic.0.000576. Epub 2017 Nov 22. Microbiology (Reading). 2017. PMID: 29165235 Free PMC article.
-
Elucidation of a novel lipid A α-(1,1)-GalA transferase gene (rgtF) from Mesorhizobium loti: Heterologous expression of rgtF causes Rhizobium etli to synthesize lipid A with α-(1,1)-GalA.Glycobiology. 2013 May;23(5):546-58. doi: 10.1093/glycob/cws223. Epub 2013 Jan 2. Glycobiology. 2013. PMID: 23283001 Free PMC article.
References
-
- Fahy E, Subramaniam S, Brown HA, Glass CK, Merrill AH, Jr., Murphy RC, Raetz CRH, Russell DW, Seyama Y, Shaw W, Shimizu T, Spener F, van Meer G, VanNieuwenhze MS, White SH, Witztum JL, Dennis EA. J. Lipid Res. 2005;46:839–862. - PubMed
-
- Raetz CRH. Annu. Rev. Biochem. 1990;59:129–170. - PubMed
-
- Raetz CRH. In: Escherichia coli and Salmonella: Cellular and Molecular Biology. 2nd Neidhardt FC, editor. American Society for Microbiology; Washington, DC: 1996. pp. 498–503.
-
- Reeves PR, Hobbs M, Valvano MA, Skurnik M, Whitfield C, Coplin D, Kido N, Klena J, Maskell D, Raetz CRH, Rick PD. Trends Microbiol. 1996;4:495–503. - PubMed
-
- Brade H, Opal SM, Vogel SN, Morrison DC. Endotoxin in Health and Disease. Marcel Dekker, Inc.; New York: 1999. p. 950.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

