Directed differentiation of mouse embryonic stem cells into thyroid follicular cells
- PMID: 16497809
- PMCID: PMC1592134
- DOI: 10.1210/en.2005-1239
Directed differentiation of mouse embryonic stem cells into thyroid follicular cells
Abstract
Elucidating the molecular mechanisms leading to the induction and specification of thyroid follicular cells is important for our understanding of thyroid development. To characterize the key events in this process, we previously established an experimental embryonic stem (ES) cell model system, which shows that wild-type mouse CCE ES cells can give rise to thyrocyte-like cells in vitro. We extend our analysis in this report by using a genetically manipulated ES cell line in which green fluorescent protein (GFP) cDNA is targeted to the TSH receptor (TSHR) gene, linking GFP expression to the transcription of the endogenous TSHR gene. The appearance of GFP-positive cells was dependent on the formation of embryoid bodies from undifferentiated ES cells and was greatly enhanced by TSH treatment during the first 2-4 d of differentiation. With the support of Matrigel, highly enriched ES cell-derived GFP-positive cells formed thyroid follicle-like clusters in a serum-free medium supplemented with TSH. Importantly, these clusters display the characteristics of thyroid follicular cells. Immunofluorescent studies confirmed the colocalization of TSHR with the Na+/I- symporter in the clusters and indicated that Na+/I- symporter was expressed exclusively in the plasma membrane. In addition, I- uptake activity was observed in these cells. Our results indicate that ES cells can be induced to differentiate into thyroid follicular cells, providing a powerful tool to study embryonic thyroid development and function.
Figures



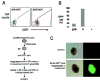

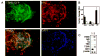
Similar articles
-
Differentiation of murine embryonic stem cells to thyrocytes requires insulin and insulin-like growth factor-1.Biochem Biophys Res Commun. 2009 Apr 3;381(2):264-70. doi: 10.1016/j.bbrc.2009.02.035. Epub 2009 Feb 14. Biochem Biophys Res Commun. 2009. PMID: 19232325 Free PMC article.
-
Committing embryonic stem cells to differentiate into thyrocyte-like cells in vitro.Endocrinology. 2003 Jun;144(6):2644-9. doi: 10.1210/en.2002-0122. Endocrinology. 2003. PMID: 12746328
-
Long Term Rescue of the TSH Receptor Knock-Out Mouse - Thyroid Stem Cell Transplantation Restores Thyroid Function.Front Endocrinol (Lausanne). 2021 Jul 2;12:706101. doi: 10.3389/fendo.2021.706101. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34276566 Free PMC article.
-
Self-renewal vs. differentiation of mouse embryonic stem cells.Biol Reprod. 2004 Dec;71(6):1755-65. doi: 10.1095/biolreprod.104.028100. Epub 2004 Aug 25. Biol Reprod. 2004. PMID: 15329329 Review.
-
New approach for the establishment of mouse early embryonic stem cells and induction of their differentiation.Hum Cell. 2001 Dec;14(4):283-91. Hum Cell. 2001. PMID: 11925930 Review.
Cited by
-
Generation and Differentiation of Adult Tissue-Derived Human Thyroid Organoids.Stem Cell Reports. 2021 Apr 13;16(4):913-925. doi: 10.1016/j.stemcr.2021.02.011. Epub 2021 Mar 11. Stem Cell Reports. 2021. PMID: 33711265 Free PMC article.
-
Stimulation of cultured h9 human embryonic stem cells with thyroid stimulating hormone does not lead to formation of thyroid-like cells.Stem Cells Int. 2012;2012:634914. doi: 10.1155/2012/634914. Epub 2012 Mar 11. Stem Cells Int. 2012. PMID: 22619683 Free PMC article.
-
Derivation of Endodermal Progenitors From Pluripotent Stem Cells.J Cell Physiol. 2015 Feb;230(2):246-58. doi: 10.1002/jcp.24771. J Cell Physiol. 2015. PMID: 25160562 Free PMC article. Review.
-
Differentiation of murine embryonic stem cells to thyrocytes requires insulin and insulin-like growth factor-1.Biochem Biophys Res Commun. 2009 Apr 3;381(2):264-70. doi: 10.1016/j.bbrc.2009.02.035. Epub 2009 Feb 14. Biochem Biophys Res Commun. 2009. PMID: 19232325 Free PMC article.
-
Thyroid follicle formation and thyroglobulin expression in multipotent endodermal stem cells.Thyroid. 2013 Apr;23(4):385-91. doi: 10.1089/thy.2012.0644. Epub 2013 Mar 18. Thyroid. 2013. PMID: 23360087 Free PMC article.
References
-
- Ambesi-Impiombato FS, Coon HG. Thyroid cells in culture. Int Rev Cytol. 1979;(Suppl):163–172. - PubMed
-
- Fayet G, Hovsepian S. Isolation of a normal human thyroid cell line: hormonal requirement for thyroglobulin regulation. Thyroid. 2002;12:539–546. - PubMed
-
- Damante G, Tell G, Di Lauro R. A unique combination of transcription factors controls differentiation of thyroid cells. Prog Nucleic Acids Res Mol Biol. 2001;66:307–356. - PubMed
-
- Tasevski V, Benn D, Peters G, Luttrell B, Simpson A. The Fischer rat thyroid cell line FRTL-5 exhibits a nondiploid karyotype. Thyroid. 1998;8:623–626. - PubMed
-
- Ealey PA, Emmerson JM, Bidey SP, Marshall NJ. Thyrotrophin stimulation of mitogenesis of the rat thyroid cell strain FRTL-5: a metaphase index assay for the detection of thyroid growth stimulators. J Endocrinol. 1985;106:203–210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

