The incidence of leukemia and mortality from sepsis in patients with severe congenital neutropenia receiving long-term G-CSF therapy
- PMID: 16497969
- PMCID: PMC1895804
- DOI: 10.1182/blood-2005-11-4370
The incidence of leukemia and mortality from sepsis in patients with severe congenital neutropenia receiving long-term G-CSF therapy
Abstract
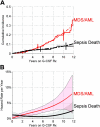
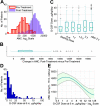
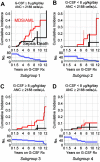
In patients with severe congenital neutropenia (SCN), sepsis mortality is reduced by treatment with granulocyte colony-stimulating factor (G-CSF), but myelodsyplastic syndrome and acute myeloid leukemia (MDS/AML) have been reported. We studied 374 patients with SCN and 29 patients with Shwachman-Diamond syndrome (SDS) on long-term G-CSF enrolled in the Severe Chronic Neutropenia International Registry. In SCN, sepsis mortality was stable at 0.9% per year. The hazard of MDS/AML increased significantly over time, from 2.9% per year after 6 years to 8.0% per year after 12 years on G-CSF. After 10 years, the cumulative incidence was 8% for sepsis mortality and 21% for MDS/AML. A subgroup of SCN patients (29%) received more than the median dose of G-CSF (> or = 8 microg/kg/d), but achieved less than the median absolute neutrophil count (ANC) response (ANC < 2.188 x 10(9)/L [2188/microL] at 6-18 months). In these less-responsive patients, the cumulative incidence of adverse events was highest: after 10 years, 40% developed MDS/AML and 14% died of sepsis, compared with 11% and 4%, respectively, of more responsive patients whose ANC was above the median on doses of G-CSF below the median. Risk of MDS/AML may be similar in SDS and SCN. In less-responsive SCN patients, early hematopoietic stem cell transplantation may be a rational option.
Figures
References
-
- Zeidler C, Welte K. Kostmann syndrome and severe congenital neutropenia. Semin Hematol. 2002;39: 82-88. - PubMed
-
- Alter BP. Inherited bone marrow failure syndromes. In: Nathan DG, Orkin SH, Look AT, Ginsburg D, eds. Hematology of Infancy and Childhood. Philadelphia: WB Saunders Inc; 2003: 280-365.
-
- Miller RW. Childhood cancer and congenital defects: a study of U.S. death certificates during the period 1960-1966. Pediatr Res. 1969;3: 389-397. - PubMed
-
- Gilman PA, Jackson DP, Guild HG. Congenital agranulocytosis: prolonged survival and terminal acute leukemia. Blood. 1970;36: 576-585. - PubMed
-
- Rosen RB, Kang SJ. Congenital agranulocytosis terminating in acute myelomonocytic leukemia. J Pediatr. 1979;94: 406-408. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

