Extracellular nucleotides and adenosine independently activate AMP-activated protein kinase in endothelial cells: involvement of P2 receptors and adenosine transporters
- PMID: 16497986
- PMCID: PMC2830086
- DOI: 10.1161/01.RES.0000215436.92414.1d
Extracellular nucleotides and adenosine independently activate AMP-activated protein kinase in endothelial cells: involvement of P2 receptors and adenosine transporters
Abstract
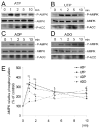
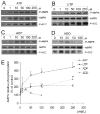
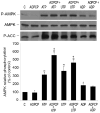
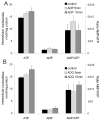
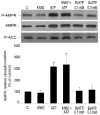
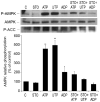
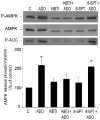
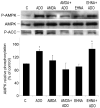
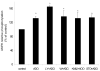
AMP-activated protein kinase (AMPK) plays a key role in the regulation of energy homeostasis and is activated in response to cellular stress, including hypoxia/ischemia and hyperglycemia. The stress events are accompanied by rapid release of extracellular nucleotides from damaged tissues or activated endothelial cells (EC) and platelets. We demonstrate that extracellular nucleotides (ATP, ADP, and UTP, but not UDP) and adenosine independently induce phosphorylation and activation of AMPK in human umbilical vein EC (HUVEC) by the mechanism that is not linked to changes in AMP:ATP ratio. HUVEC express NTPDases, as well as 5'-nucleotidase; hence, nucleotides can be metabolized to adenosine. However, inhibition of 5'-nucleotidase had no effect on ATP/ADP/UTP-induced phospho- rylation of AMPK, indicating that AMPK activation occurred as a direct response to nucleotides. Nucleotide-evoked phosphorylation of AMPK in HUVEC was mediated by P2Y1, P2Y2, and/or P2Y4 receptors, whereas P2Y6, P2Y11, and P2X receptors were not involved. The nucleotide-induced phosphorylation of AMPK was affected by changes in the concentration of intracellular Ca2+ and by Ca2+/calmodulin-dependent kinase kinase (CaMKK), although most likely it was not dependent on LKB1 kinase. Adenosine-induced phosphorylation of AMPK was not mediated by P1 receptors but required adenosine uptake by equilibrative nucleoside transporters followed by its (intracellular) metabolism to AMP. Moreover, adenosine effect was Ca2+ and CaMKK independent, although probably associated with upstream LKB1. We hypothesize that P2 receptors and adenosine transporters could be novel targets for the pharmacological regulation of AMPK activity and its downstream effects on EC function.
Figures


References
-
- Hardie DG, Salt IP, Davies SP. Analysis of the role of the AMP-activated protein kinase in the response to cellular stress. Methods Mol Biol. 2000;99:63–74. - PubMed
-
- Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem. 1996;271:27879–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

