ICER induced by hyperglycemia represses the expression of genes essential for insulin exocytosis
- PMID: 16498408
- PMCID: PMC1409716
- DOI: 10.1038/sj.emboj.7601008
ICER induced by hyperglycemia represses the expression of genes essential for insulin exocytosis
Abstract
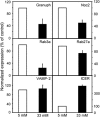
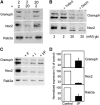
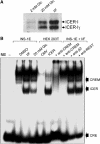
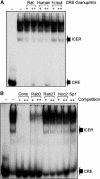
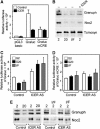
The GTPases Rab3a and Rab27a and their effectors Granuphilin/Slp4 and Noc2 are essential regulators of neuroendocrine secretion. Chronic exposure of pancreatic beta-cells to supraphysiological glucose levels decreased selectively the expression of these proteins. This glucotoxic effect was mimicked by cAMP-raising agents and blocked by PKA inhibitors. We demonstrate that the transcriptional repressor ICER, which is induced in a PKA-dependent manner by chronic hyperglycemia and cAMP-raising agents, is responsible for the decline of the four genes. ICER overexpression diminished the level of Granuphilin, Noc2, Rab3a and Rab27a by binding to cAMP responsive elements located in the promoters of these genes and inhibited exocytosis of beta-cells in response to secretagogues. Moreover, the loss in the expression of the genes of the secretory machinery caused by glucose and cAMP-raising agents was prevented by an antisense construct that reduces ICER levels. We propose that induction of inappropriate ICER levels lead to defects in the secretory process of pancreatic beta-cells possibly contributing, in conjunction with other known deleterious effects of hyperglycemia, to defective insulin release in type 2 diabetes.
Figures




References
-
- Allagnat F, Martin D, Condorelli DF, Waeber G, Haefliger JA (2005) Glucose represses connexin36 in insulin-secreting cells. J Cell Sci 118: 5335–5344 - PubMed
-
- Briaud I, Lingohr MK, Dickson LM, Wrede CE, Rhodes CJ (2003) Differential activation mechanisms of Erk-1/2 and p70(S6K) by glucose in pancreatic beta-cells. Diabetes 52: 974–983 - PubMed
-
- Cheviet S, Coppola T, Haynes LP, Burgoyne RD, Regazzi R (2004a) The Rab-binding protein Noc2 is associated with insulin-containing secretory granules and is essential for pancreatic beta-cell exocytosis. Mol Endocrinol 18: 117–126 - PubMed
-
- Cheviet S, Waselle L, Regazzi R (2004b) Noc-king out exocrine and endocrine secretion. Trends Cell Biol 14: 525–528 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

