Complement: a novel factor in basal and ischemia-induced neurogenesis
- PMID: 16498410
- PMCID: PMC1422160
- DOI: 10.1038/sj.emboj.7601004
Complement: a novel factor in basal and ischemia-induced neurogenesis
Abstract
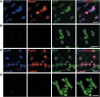
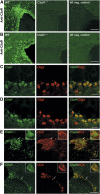
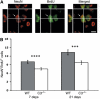
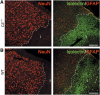
Through its involvement in inflammation, opsonization, and cytolysis, the complement protects against infectious agents. Although most of the complement proteins are synthesized in the central nervous system (CNS), the role of the complement system in the normal or ischemic CNS remains unclear. Here we demonstrate for the first time that neural progenitor cells and immature neurons express receptors for complement fragments C3a and C5a (C3a receptor (C3aR) and C5a receptor). Mice that are deficient in complement factor C3 (C3(-/-)) lack C3a and are unable to generate C5a through proteolytic cleavage of C5 by C5-convertase. Intriguingly, basal neurogenesis is decreased both in C3(-/-) mice and in mice lacking C3aR or mice treated with a C3aR antagonist. The C3(-/-) mice had impaired ischemia-induced neurogenesis both in the subventricular zone, the main source of neural progenitor cells in adult brain, and in the ischemic region, despite normal proliferative response and larger infarct volumes. Thus, in the adult mammalian CNS, complement activation products promote both basal and ischemia-induced neurogenesis.
Figures






Similar articles
-
Signaling through C5aR is not involved in basal neurogenesis.J Neurosci Res. 2007 Oct;85(13):2892-7. doi: 10.1002/jnr.21401. J Neurosci Res. 2007. PMID: 17551982
-
Targeting C3a/C5a receptors inhibits human mesangial cell proliferation and alleviates immunoglobulin A nephropathy in mice.Clin Exp Immunol. 2017 Jul;189(1):60-70. doi: 10.1111/cei.12961. Epub 2017 Apr 10. Clin Exp Immunol. 2017. PMID: 28295247 Free PMC article.
-
Mast cell anaphylatoxin receptor expression can enhance IgE-dependent skin inflammation in mice.J Allergy Clin Immunol. 2013 Feb;131(2):541-8.e1-9. doi: 10.1016/j.jaci.2012.05.009. Epub 2012 Jun 22. J Allergy Clin Immunol. 2013. PMID: 22728083 Free PMC article.
-
The Complement C3a and C5a Signaling in Renal Diseases: A Bridge between Acute and Chronic Inflammation.Nephron. 2024;148(10):712-723. doi: 10.1159/000538241. Epub 2024 Mar 8. Nephron. 2024. PMID: 38452744 Review.
-
Evolution of anaphylatoxins, their diversity and novel roles in innate immunity: insights from the study of fish complement.Vet Immunol Immunopathol. 2005 Oct 18;108(1-2):77-89. doi: 10.1016/j.vetimm.2005.07.009. Vet Immunol Immunopathol. 2005. PMID: 16112742 Review.
Cited by
-
Serum complement C3 and islet β-cell function in patients with type 2 diabetes: A 4.6-year prospective follow-up study.Endocrine. 2020 Feb;67(2):321-330. doi: 10.1007/s12020-019-02144-z. Epub 2019 Nov 30. Endocrine. 2020. PMID: 31786774
-
The C5a anaphylatoxin receptor CD88 is expressed in presynaptic terminals of hippocampal mossy fibres.J Neuroinflammation. 2009 Nov 16;6:34. doi: 10.1186/1742-2094-6-34. J Neuroinflammation. 2009. PMID: 19917081 Free PMC article.
-
Significance of Complement System in Ischemic Stroke: A Comprehensive Review.Aging Dis. 2019 Apr 1;10(2):429-462. doi: 10.14336/AD.2019.0119. eCollection 2019 Apr. Aging Dis. 2019. PMID: 31011487 Free PMC article. Review.
-
PMX53 protects spinal cord from ischemia-reperfusion injury in rats in the short term.Spinal Cord. 2016 Apr;54(4):254-8. doi: 10.1038/sc.2015.146. Epub 2015 Sep 8. Spinal Cord. 2016. PMID: 26345482
-
C3a Enhances the Formation of Intestinal Organoids through C3aR1.Front Immunol. 2017 Sep 4;8:1046. doi: 10.3389/fimmu.2017.01046. eCollection 2017. Front Immunol. 2017. PMID: 28928734 Free PMC article.
References
-
- Akita N, Nakase H, Kaido T, Kanemoto Y, Sakaki T (2003) Protective effect of C1 esterase inhibitor on reperfusion injury in the rat middle cerebral artery occlusion model. Neurosurgery 52: 395–400 - PubMed
-
- Ames RS, Lee D, Foley JJ, Jurewicz AJ, Tornetta MA, Bautsch W, Settmacher B, Klos A, Erhard KF, Cousins RD, Sulpizio AC, Hieble JP, McCafferty G, Ward KW, Adams JL, Bondinell WE, Underwood DC, Osborn RR, Badger AM, Sarau HM (2001) Identification of a selective nonpeptide antagonist of the anaphylatoxin C3a receptor that demonstrates antiinflammatory activity in animal models. J Immunol 166: 6341–6348 - PubMed
-
- Arnett HA, Fancy SP, Alberta JA, Zhao C, Plant SR, Kaing S, Raine CS, Rowitch DH, Franklin RJ, Stiles CD (2004) bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science 306: 2111–2115 - PubMed
-
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O (2002) Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 8: 963–970 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

