Structural and functional insights into the B30.2/SPRY domain
- PMID: 16498413
- PMCID: PMC1422157
- DOI: 10.1038/sj.emboj.7600994
Structural and functional insights into the B30.2/SPRY domain
Abstract
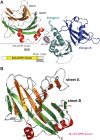
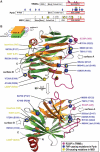
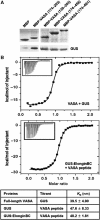
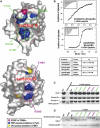
The B30.2/SPRY domain is present in approximately 700 eukaryotic (approximately 150 human) proteins, including medically important proteins such as TRIM5alpha and Pyrin. Nonetheless, the functional role of this modular domain remained unclear. Here, we report the crystal structure of an SPRY-SOCS box family protein GUSTAVUS in complex with Elongins B and C, revealing a highly distorted two-layered beta-sandwich core structure of its B30.2/SPRY domain. Ensuing studies identified one end of the beta-sandwich as the surface interacting with an RNA helicase VASA with a 40 nM dissociation constant. The sequence variation in TRIM5alpha responsible for HIV-1 restriction and most of the mutations in Pyrin causing familial Mediterranean fever map on this surface, implicating the corresponding region in many B30.2/SPRY domains as the ligand-binding site. The amino acids lining the binding surface are highly variable among the B30.2/SPRY domains, suggesting that these domains are protein-interacting modules, which recognize a specific individual partner protein rather than a consensus sequence motif.
Figures

References
-
- Bakkaloglu A (2003) Familial Mediterranean fever. Pediatr Nephrol 18: 853–859 - PubMed
-
- Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL (1998) Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr 54: 905–921 - PubMed
-
- Carrera P, Johnstone O, Nakamura A, Casanova J, Jackle H, Lasko P (2000) VASA mediates translation through interaction with a Drosophila yIF2 homolog. Mol Cell 5: 181–187 - PubMed
-
- Cuff JA, Clamp ME, Siddiqui AS, Finlay M, Barton GJ (1998) JPred: a consensus secondary structure prediction server. Bioinformatics 14: 892–893 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

