Activation energies for dissociation of double strand oligonucleotide anions: evidence for watson-crick base pairing in vacuo
- PMID: 16498487
- PMCID: PMC1380309
- DOI: 10.1021/ja973534h
Activation energies for dissociation of double strand oligonucleotide anions: evidence for watson-crick base pairing in vacuo
Abstract
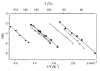
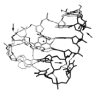
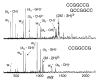
The dissociation kinetics of a series of complementary and noncomplementary DNA duplexes, (TGCA)(2) (3-), (CCGG)(2) (3-), (AATTAAT)(2) (3-), (CCGGCCG)(2) (3-), A(7)*T(7) (3-), A(7)*A(7) (3-), T(7)*T(7) (3-), and A(7)*C(7) (3-) were investigated using blackbody infrared radiative dissociation in a Fourier transform mass spectrometer. From the temperature dependence of the unimolecular dissociation rate constants, Arrhenius activation parameters in the zero-pressure limit are obtained. Activation energies range from 1.2 to 1.7 eV, and preexponential factors range from 10(13) to 10(19) s(-1). Dissociation of the duplexes results in cleavage of the noncovalent bonds and/or cleavage of covalent bonds leading to loss of a neutral nucleobase followed by backbone cleavage producing sequence-specific (a - base) and w ions. Four pieces of evidence are presented which indicate that Watson-Crick (WC) base pairing is preserved in complementary DNA duplexes in the gas phase: i. the activation energy for dissociation of the complementary dimer, A(7)*T(7) (3-), to the single strands is significantly higher than that for the related noncomplementary A(7)*A(7) (3-) and T(7)*T(7) (3-) dimers, indicating a stronger interaction between strands with a specific base sequence, ii. extensive loss of neutral adenine occurs for A(7)*A(7) (3-) and A(7)*C(7) (3-) but not for A(7)*T(7) (3-) consistent with this process being shut down by WC hydrogen bonding, iii. a correlation is observed between the measured activation energy for dissociation to single strands and the dimerization enthalpy (-DeltaH(d)) in solution, and iv. molecular dynamics carried out at 300 and 400 K indicate that WC base pairing is preserved for A(7)*T(7) (3-) duplex, although the helical structure is essentially lost. In combination, these results provide strong evidence that WC base pairing can exist in the complete absence of solvent.
Figures

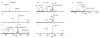

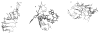


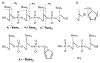
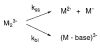
References
-
- Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Science. 1989;246:64–71. - PubMed
-
- Hillenkamp F, Karas M, Beavis RC, Chait BT. Anal Chem. 1991;63:1193A–1202A. - PubMed
-
- Bowers MT, Marshall AG, McLafferty FW. J Phys Chem. 1996;100:12897–12910.
-
- McLafferty FW. Acc Chem Res. 1994;27:379–386. and references therein.
- Kelleher NL, Senko MW, Siegel MM, McLafferty FW. J Am Soc Mass Spectrom. 1997;8:380–383.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
