Cholinergic dysfunction in a mouse model of Alzheimer disease is reversed by an anti-A beta antibody
- PMID: 16498501
- PMCID: PMC1378188
- DOI: 10.1172/JCI27120
Cholinergic dysfunction in a mouse model of Alzheimer disease is reversed by an anti-A beta antibody
Abstract
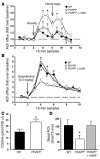
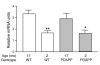
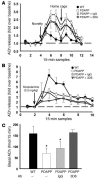
Disruption of cholinergic neurotransmission contributes to the memory impairment that characterizes Alzheimer disease (AD). Since the amyloid cascade hypothesis of AD pathogenesis postulates that amyloid beta (A beta) peptide accumulation in critical brain regions also contributes to memory impairment, we assessed cholinergic function in transgenic mice where the human A beta peptide is overexpressed. We first measured hippocampal acetylcholine (ACh) release in young, freely moving PDAPP mice, a well-characterized transgenic mouse model of AD, and found marked A beta-dependent alterations in both basal and evoked ACh release compared with WT controls. We also found that A beta could directly interact with the high-affinity choline transporter which may impair steady-state and on-demand ACh release. Treatment of PDAPP mice with the anti-A beta antibody m266 rapidly and completely restored hippocampal ACh release and high-affinity choline uptake while greatly reducing impaired habituation learning that is characteristic of these mice. Thus, soluble "cholinotoxic" species of the A beta peptide can directly impair cholinergic neurotransmission in PDAPP mice leading to memory impairment in the absence of overt neurodegeneration. Treatment with certain anti-A beta antibodies may therefore rapidly reverse this cholinergic dysfunction and relieve memory deficits associated with early AD.
Figures


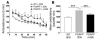
References
-
- Perry E, Walker M, Grace J, Perry R. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci. 1999;22:273–280. - PubMed
-
- Bartus R, Dean RL, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–411. - PubMed
-
- Coyle JT, Price DL, DeLong MR. Alzheimer’s disease: a disorder of cortical cholinergic innervation. Science. 1983;219:1184–1190. - PubMed
-
- Whitehouse PJ, et al. Alzheimer disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann. Neurol. 1981;10:122–126. - PubMed
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

