The cell cycle: a critical therapeutic target to prevent vascular proliferative disease
- PMID: 16498512
- PMCID: PMC2780832
- DOI: 10.1016/s0828-282x(06)70986-2
The cell cycle: a critical therapeutic target to prevent vascular proliferative disease
Abstract
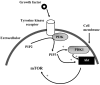
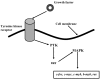
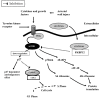
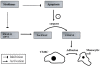
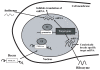
Percutaneous coronary intervention is the preferred revascularization approach for most patients with coronary artery disease. However, this strategy is limited by renarrowing of the vessel by neointimal hyperplasia within the stent lumen (in-stent restenosis). Vascular smooth muscle cell proliferation is a major component in this healing process. This process is mediated by multiple cytokines and growth factors, which share a common pathway in inducing cell proliferation: the cell cycle. The cell cycle is highly regulated by numerous mechanisms ensuring orderly and coordinated cell division. The present review discusses current concepts related to regulation of the cell cycle and new therapeutic options that target aspects of the cell cycle.
L’intervention coronaire percutanée est la démarche de revascularisation de choix pour la plupart des patients atteints d’une coronaropathie. Cependant, cette stratégie est limitée par un nouveau rétrécissement du vaisseau imputable à une hyperplasie néo-intimale dans la lumière de l’endoprothèse (une resténose dans l’endoprothèse). La prolifération des cellules vasculaires des muscles lisses est un élément important de ce processus de guérison. Ce processus est médié par de multiples cytokines et facteurs de croissance, qui partagent une voie commune dans l’induction de la prolifération cellulaire : le cycle cellulaire. Le cycle cellulaire est hautement régularisé par de nombreux mécanismes qui assurent une division cellulaire ordonnée et coordonnée. La présente analyse porte sur les concepts courants reliés à la régulation du cycle cellulaire et sur de nouvelles options thérapeutiques qui ciblent des aspects du cycle cellulaire.
Figures

References
-
- Tunstall-Pedoe H, Kuulasmaa K, Mahonen M, Tolonen H, Ruokokoski E, Amouyel P. Contribution of trends in survival and coronary-event rates to changes in coronary heart disease mortality: 10-year results from 37 WHO MONICA project populations. Monitoring trends and determinants in cardiovascular disease. Lancet. 1999;353:1547–57. - PubMed
-
- Mintz GS, Popma JJ, Pichard AD, et al. Arterial remodeling after coronary angioplasty: A serial intravascular ultrasound study. Circulation. 1996;94:35–43. - PubMed
-
- Hoffmann R, Mintz GS, Dussaillant GR, et al. Patterns and mechanisms of in-stent restenosis. A serial intravascular ultrasound study. Circulation. 1996;94:1247–54. - PubMed
-
- Wilensky RL, March KL, Gradus-Pizlo I, Sandusky G, Fineberg N, Hathaway DR. Vascular injury, repair, and restenosis after percutaneous transluminal angioplasty in the atherosclerotic rabbit. Circulation. 1995;92:2995–3005. - PubMed
-
- Wong A, Chan C. Drug-eluting stents: The end of restenosis? Ann Acad Med Singapore. 2004;33:423–31. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

