Crystal structure of hypothetical protein YfiH from Shigella flexneri at 2 A resolution
- PMID: 16498617
- PMCID: PMC2792012
- DOI: 10.1002/prot.20589
Crystal structure of hypothetical protein YfiH from Shigella flexneri at 2 A resolution
Figures
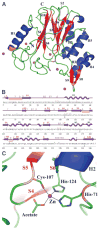

Similar articles
-
Structure of YidB protein from Shigella flexneri shows a new fold with homeodomain motif.Proteins. 2006 Nov 1;65(2):509-13. doi: 10.1002/prot.21054. Proteins. 2006. PMID: 16927377 Free PMC article. No abstract available.
-
Structure of Spa15, a type III secretion chaperone from Shigella flexneri with broad specificity.EMBO Rep. 2004 May;5(5):477-83. doi: 10.1038/sj.embor.7400144. Epub 2004 Apr 16. EMBO Rep. 2004. PMID: 15088068 Free PMC article.
-
Novel fold of VirA, a type III secretion system effector protein from Shigella flexneri.Protein Sci. 2008 Dec;17(12):2167-73. doi: 10.1110/ps.037978.108. Epub 2008 Sep 11. Protein Sci. 2008. PMID: 18787201 Free PMC article.
-
Crystal structure of a glutamate/aspartate binding protein complexed with a glutamate molecule: structural basis of ligand specificity at atomic resolution.J Mol Biol. 2008 Sep 26;382(1):99-111. doi: 10.1016/j.jmb.2008.06.091. Epub 2008 Jul 9. J Mol Biol. 2008. PMID: 18640128
-
Crystal structure and functional characterization of SF216 from Shigella flexneri.FEBS Lett. 2017 Nov;591(21):3692-3703. doi: 10.1002/1873-3468.12873. Epub 2017 Oct 17. FEBS Lett. 2017. PMID: 28983914
Cited by
-
C13orf31 (FAMIN) is a central regulator of immunometabolic function.Nat Immunol. 2016 Sep;17(9):1046-56. doi: 10.1038/ni.3532. Epub 2016 Aug 1. Nat Immunol. 2016. PMID: 27478939 Free PMC article.
-
Enhanced discovery of bacterial laccase-like multicopper oxidase through computer simulation and metagenomic analysis of industrial wastewater.FEBS Open Bio. 2025 Jul;15(7):1090-1102. doi: 10.1002/2211-5463.70037. Epub 2025 Apr 15. FEBS Open Bio. 2025. PMID: 40230330 Free PMC article.
-
Isolation, Purification, and Characterization of a Laccase-Degrading Aflatoxin B1 from Bacillus amyloliquefaciens B10.Toxins (Basel). 2022 Mar 31;14(4):250. doi: 10.3390/toxins14040250. Toxins (Basel). 2022. PMID: 35448859 Free PMC article.
-
Structural Basis for the Peptidoglycan-Editing Activity of YfiH.mBio. 2021 Feb 22;13(1):e0364621. doi: 10.1128/mbio.03646-21. Epub 2022 Feb 15. mBio. 2021. PMID: 35164571 Free PMC article.
-
A conserved editing mechanism for the fidelity of bacterial cell wall biosynthesis.Proc Natl Acad Sci U S A. 2025 Jul 15;122(28):e2505676122. doi: 10.1073/pnas.2505676122. Epub 2025 Jul 9. Proc Natl Acad Sci U S A. 2025. PMID: 40632566
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

