The role of synaptic reorganization in mesial temporal lobe epilepsy
- PMID: 16500154
- PMCID: PMC2829602
- DOI: 10.1016/j.yebeh.2006.01.011
The role of synaptic reorganization in mesial temporal lobe epilepsy
Abstract
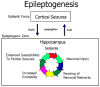
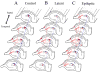
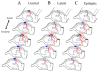
The mechanisms underlying mesial temporal lobe epilepsy (MTLE) remain uncertain. Putative mechanisms should account for several features characteristic of the clinical presentation and the neurophysiological and neuropathological abnormalities observed in patients with intractable MTLE. Synaptic reorganization of the mossy fiber pathway has received considerable attention over the past two decades as a potential mechanism that increases the excitability of the hippocampal network through the formation of new recurrent excitatory collaterals. Morphological plasticity beyond the mossy fiber pathway has not been as thoroughly investigated. Recently, plasticity of the CA1 pyramidal axons has been demonstrated in acute and chronic experimental models of MTLE. As the hippocampal formation is topographically organized in stacks of slices (lamellae), synaptic reorganization of CA1 axons projecting to subiculum appears to increase the connectivity between lamellae, providing a mechanism for translamellar synchronization of cellular hyperexcitability, leading to pharmacologically intractable seizures.
Figures


Similar articles
-
Sprouting and synaptic reorganization in the subiculum and CA1 region of the hippocampus in acute and chronic models of partial-onset epilepsy.Neuroscience. 2004;126(3):677-88. doi: 10.1016/j.neuroscience.2004.04.014. Neuroscience. 2004. PMID: 15183517 Free PMC article.
-
[Correlation between hippocampal mossy fiber sprouting and synaptic reorganization and mechanisms of temporal lobe epilepsy].Zhonghua Yi Xue Za Zhi. 2007 Jan 30;87(5):341-4. Zhonghua Yi Xue Za Zhi. 2007. PMID: 17456365 Chinese.
-
Synaptic reorganization in subiculum and CA3 after early-life status epilepticus in the kainic acid rat model.Epilepsy Res. 2007 Feb;73(2):156-65. doi: 10.1016/j.eplepsyres.2006.09.004. Epub 2006 Oct 27. Epilepsy Res. 2007. PMID: 17070016 Free PMC article.
-
Experimental models of temporal lobe epilepsy: new insights from the study of kindling and synaptic reorganization.Epilepsia. 1990;31 Suppl 3:S45-54. doi: 10.1111/j.1528-1157.1990.tb05859.x. Epilepsia. 1990. PMID: 2226371 Review.
-
Selective degeneration and synaptic reorganization of hippocampal interneurons in a chronic model of temporal lobe epilepsy.Adv Neurol. 2006;97:69-76. Adv Neurol. 2006. PMID: 16383116 Review.
Cited by
-
Non-Coding RNAs: New Biomarkers and Therapeutic Targets for Temporal Lobe Epilepsy.Int J Mol Sci. 2022 Mar 11;23(6):3063. doi: 10.3390/ijms23063063. Int J Mol Sci. 2022. PMID: 35328484 Free PMC article. Review.
-
Scoping review of disease-modifying effect of drugs in experimental epilepsy.Front Neurol. 2023 Feb 23;14:1097473. doi: 10.3389/fneur.2023.1097473. eCollection 2023. Front Neurol. 2023. PMID: 36908628 Free PMC article.
-
Thrombospondins as key regulators of synaptogenesis in the central nervous system.Matrix Biol. 2012 Apr;31(3):170-7. doi: 10.1016/j.matbio.2012.01.004. Epub 2012 Jan 21. Matrix Biol. 2012. PMID: 22285841 Free PMC article. Review.
-
The Potential Role of MicroRNA-124 in Cerebral Ischemia Injury.Int J Mol Sci. 2019 Dec 23;21(1):120. doi: 10.3390/ijms21010120. Int J Mol Sci. 2019. PMID: 31878035 Free PMC article. Review.
-
Conventional anticonvulsant drugs in the guinea-pig kindling model of partial seizures: effects of repeated administration.Exp Brain Res. 2007 Mar;178(1):115-25. doi: 10.1007/s00221-006-0716-z. Epub 2007 Jan 26. Exp Brain Res. 2007. PMID: 17256170
References
-
- Amaral DG, Witter M. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–91. - PubMed
-
- Anderson P, Bliss TV, Skrede KK. Lamellar organization of hippocampal excitatory pathways. Exp Brain Res. 1971;13:222–38. - PubMed
-
- Andersen P, Bland BH, Dudar JD. Organization of the hippocampal output. Exp Brain Res. 1973;17:152–68. - PubMed
-
- Andersen P, Soleng AF, Raastad M. The hippocampal lamella hypothesis revisited. Brain Res. 2000;886:165–71. - PubMed
-
- Babb TL, Lieb JP, Brown WJ, Pretorius J, Crandall PH. Distribution of pyramidal cell density and hyperexcitability in the epileptic human hippocampal formation. Epilepsia. 1984;25:721–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

