Acute lung infections in normal and immunocompromised hosts
- PMID: 16500210
- PMCID: PMC7119122
- DOI: 10.1016/j.rcl.2005.10.009
Acute lung infections in normal and immunocompromised hosts
Abstract
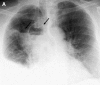
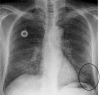
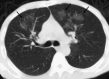
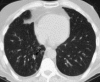
Pulmonary infections are among the most common causes of morbidity and mortality worldwide, and contribute substantially to annual medical expenditures in the United States. Despite the availability of antimicrobial agents, pneumonia constitutes the sixth most common cause of death and the number one cause of death from infection. Pneumonia can be particularly life-threatening in the elderly, in individuals who have pre-existing heart and lung conditions, in patients who have suppressed or weakened immunity, and in pregnant women. This article discusses some of the important causes of acute lung infections in normal and immunocompromised hosts. Because there often is considerable overlap, infections are categorized by the host immune status that is most likely to be associated with a particular pathogen.
Figures
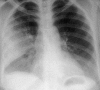
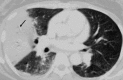
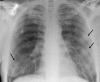
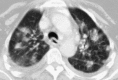
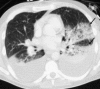
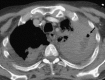
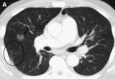

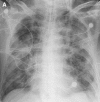




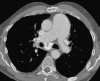
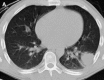

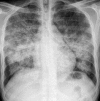
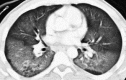
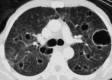
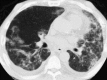
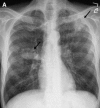

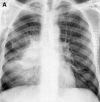






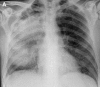



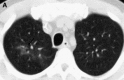

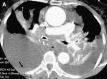


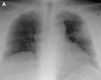

Similar articles
-
Imaging of opportunistic fungal infections in immunocompromised patient.Eur J Radiol. 2004 Aug;51(2):130-8. doi: 10.1016/j.ejrad.2004.03.007. Eur J Radiol. 2004. PMID: 15246518
-
[Radiographic imaging of bacterial pneumonia].Nihon Rinsho. 2007 Feb 28;65 Suppl 2 Pt. 1:231-4. Nihon Rinsho. 2007. PMID: 17455622 Review. Japanese. No abstract available.
-
Lung infections: the radiologist's perspective.Diagn Interv Imaging. 2012 Jun;93(6):431-40. doi: 10.1016/j.diii.2012.04.021. Epub 2012 May 31. Diagn Interv Imaging. 2012. PMID: 22658280 Review.
-
Pneumonia: ... and to tuberculosis as differential diagnosis in community acquired pneumonia.BMJ. 2006 May 20;332(7551):1214. doi: 10.1136/bmj.332.7551.1214-a. BMJ. 2006. PMID: 16710010 Free PMC article. No abstract available.
-
Viral and Pneumocystis carinii infections of the lung in the immunocompromised host.J Thorac Imaging. 1999 Jan;14(1):25-36. doi: 10.1097/00005382-199901000-00003. J Thorac Imaging. 1999. PMID: 9894951 Review.
Cited by
-
Spontaneous esophageal perforation in a patient with mixed connective tissue disease.Open Rheumatol J. 2011;5:138-43. doi: 10.2174/1874312901105010138. Epub 2011 Dec 30. Open Rheumatol J. 2011. PMID: 22279514 Free PMC article.
-
Pneumonia caused by Moraxella catarrhalis in haematopoietic stem cell transplant patients. Report of two cases and review of the literature.Libyan J Med. 2007 Sep 1;2(3):144-7. doi: 10.4176/070424. Libyan J Med. 2007. PMID: 21503215 Free PMC article.
-
Spectrum of imaging findings in pulmonary infections. Part 1: Bacterial and viral.Pol J Radiol. 2019 Apr 18;84:e205-e213. doi: 10.5114/pjr.2019.85812. eCollection 2019. Pol J Radiol. 2019. PMID: 31481992 Free PMC article. Review.
-
Simultaneous Streptococcus pneumoniae empyema in fraternal twins.IDCases. 2017 Apr 26;9:9-11. doi: 10.1016/j.idcr.2017.04.014. eCollection 2017. IDCases. 2017. PMID: 28529888 Free PMC article.
-
Unveiling the Unexpected: An Intriguing Case of Neonatal Nocardial Pneumonia.J Indian Assoc Pediatr Surg. 2025 Mar-Apr;30(2):225-228. doi: 10.4103/jiaps.jiaps_121_24. Epub 2025 Mar 3. J Indian Assoc Pediatr Surg. 2025. PMID: 40191495 Free PMC article.
References
-
- Reittner P., Ward S., Heyneman L. Pneumonia: high-resolution CT findings in 114 patients. Eur Radiol. 2003;13(3):515–521. - PubMed
-
- Webb W.R., Higgins C.B. Thoracic imaging-pulmonary and cardiovascular radiology. Lippincott Williams and Wilkens; Philadelphia: 2005.
-
- American Thoracic Society Guidelines for the initial management of adults with community acquired pneumonia: diagnosis, assessment of severity, and initial antimicrobial therapy. Am J Respir Crit Care Med. 2001;163:1730–1754. - PubMed
-
- National Center for Health Statistics National hospital discharge survey: annual summary 1990. Vital Health Stat. 1998;13:1–225. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

