Orientation of a beta-hairpin antimicrobial peptide in lipid bilayers from two-dimensional dipolar chemical-shift correlation NMR
- PMID: 16500957
- PMCID: PMC1440742
- DOI: 10.1529/biophysj.105.062075
Orientation of a beta-hairpin antimicrobial peptide in lipid bilayers from two-dimensional dipolar chemical-shift correlation NMR
Abstract
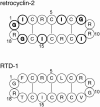
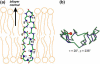
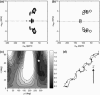
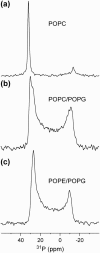
The orientation of a beta-sheet membrane peptide in lipid bilayers is determined, for the first time, using two-dimensional (2D) (15)N solid-state NMR. Retrocyclin-2 is a disulfide-stabilized cyclic beta-hairpin peptide with antibacterial and antiviral activities. We used 2D separated local field spectroscopy correlating (15)N-(1)H dipolar coupling with (15)N chemical shift to determine the orientation of multiply (15)N-labeled retrocyclin-2 in uniaxially aligned phosphocholine bilayers. Calculated 2D spectra exhibit characteristic resonance patterns that are sensitive to both the tilt of the beta-strand axis and the rotation of the beta-sheet plane from the bilayer normal and that yield resonance assignment without the need for singly labeled samples. Retrocyclin-2 adopts a transmembrane orientation in dilauroylphosphatidylcholine bilayers, with the strand axis tilted at 20 degrees +/- 10 degrees from the bilayer normal, but changes to a more in-plane orientation in thicker 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidyl-choline (POPC) bilayers with a tilt angle of 65 degrees +/- 15 degrees . These indicate that hydrophobic mismatch regulates the peptide orientation. The 2D spectra are sensitive not only to the peptide orientation but also to its backbone (phi, psi) angles. Neither a bent hairpin conformation, which is populated in solution, nor an ideal beta-hairpin with uniform (phi, psi) angles and coplanar strands, agrees with the experimental spectrum. Thus, membrane binding orders the retrocyclin conformation by reducing the beta-sheet curvature but does not make it ideal. (31)P NMR spectra of lipid bilayers with different compositions indicate that retrocyclin-2 selectively disrupts the orientational order of anionic membranes while leaving zwitteronic membranes intact. These structural results provide insights into the mechanism of action of this beta-hairpin antimicrobial peptide.
Figures



Similar articles
-
Structure and mechanism of beta-hairpin antimicrobial peptides in lipid bilayers from solid-state NMR spectroscopy.Mol Biosyst. 2009 Apr;5(4):317-22. doi: 10.1039/b820398a. Epub 2009 Jan 27. Mol Biosyst. 2009. PMID: 19396367 Free PMC article. Review.
-
Solid-state NMR investigation of the selective perturbation of lipid bilayers by the cyclic antimicrobial peptide RTD-1.Biochemistry. 2004 Aug 3;43(30):9800-12. doi: 10.1021/bi036243w. Biochemistry. 2004. PMID: 15274634
-
Solid-state NMR investigations of peptide-lipid interaction and orientation of a beta-sheet antimicrobial peptide, protegrin.Biochemistry. 2002 Aug 6;41(31):9852-62. doi: 10.1021/bi0257991. Biochemistry. 2002. PMID: 12146951
-
Membrane-bound conformation and topology of the antimicrobial peptide tachyplesin I by solid-state NMR.Biochemistry. 2006 Nov 7;45(44):13323-30. doi: 10.1021/bi061424u. Biochemistry. 2006. PMID: 17073453
-
Peptide-lipid interactions of the beta-hairpin antimicrobial peptide tachyplesin and its linear derivatives from solid-state NMR.Biochim Biophys Acta. 2006 Sep;1758(9):1285-91. doi: 10.1016/j.bbamem.2006.03.016. Epub 2006 Apr 5. Biochim Biophys Acta. 2006. PMID: 16678119 Review.
Cited by
-
Effects of arginine density on the membrane-bound structure of a cationic antimicrobial peptide from solid-state NMR.Biochim Biophys Acta. 2009 Feb;1788(2):514-21. doi: 10.1016/j.bbamem.2008.10.027. Epub 2008 Nov 14. Biochim Biophys Acta. 2009. PMID: 19059201 Free PMC article.
-
High-resolution orientation and depth of insertion of the voltage-sensing S4 helix of a potassium channel in lipid bilayers.J Mol Biol. 2010 Aug 27;401(4):642-52. doi: 10.1016/j.jmb.2010.06.048. Epub 2010 Jun 30. J Mol Biol. 2010. PMID: 20600109 Free PMC article.
-
The cyclic cystine ladder in θ-defensins is important for structure and stability, but not antibacterial activity.J Biol Chem. 2013 Apr 12;288(15):10830-40. doi: 10.1074/jbc.M113.451047. Epub 2013 Feb 21. J Biol Chem. 2013. PMID: 23430740 Free PMC article.
-
Structure and mechanism of beta-hairpin antimicrobial peptides in lipid bilayers from solid-state NMR spectroscopy.Mol Biosyst. 2009 Apr;5(4):317-22. doi: 10.1039/b820398a. Epub 2009 Jan 27. Mol Biosyst. 2009. PMID: 19396367 Free PMC article. Review.
-
On the role of NMR spectroscopy for characterization of antimicrobial peptides.Methods Mol Biol. 2013;1063:159-80. doi: 10.1007/978-1-62703-583-5_9. Methods Mol Biol. 2013. PMID: 23975777 Free PMC article. Review.
References
-
- Wang, J., J. Denny, C. Tian, S. Kim, Y. Mo, F. Kovacs, Z. Song, K. Nishimura, Z. Gan, R. Fu, J. R. Quine, and T. A. Cross. 2000. Imaging membrane protein helical wheels. J. Magn. Reson. 144:162–167. - PubMed
-
- Vosegaard, T., and N. C. Nielsen. 2002. Towards high-resolution solid-state NMR on large uniformly 15N- and [13C,15N]-labeled membrane proteins in oriented lipid bilayers. J. Biomol. NMR. 22:225–247. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

