Probing perturbation of bovine lung surfactant extracts by albumin using DSC and 2H-NMR
- PMID: 16500977
- PMCID: PMC1440744
- DOI: 10.1529/biophysj.105.077370
Probing perturbation of bovine lung surfactant extracts by albumin using DSC and 2H-NMR
Abstract
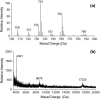
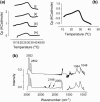
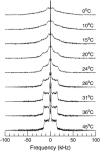
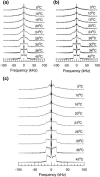
Lung surfactant (LS), a lipid-protein mixture, forms films at the lung air-water interface and prevents alveolar collapse at end expiration. In lung disease and injury, the surface activity of LS is inhibited by leakage of serum proteins such as albumin into the alveolar hypophase. Multilamellar vesicular dispersions of a clinically used replacement, bovine lipid extract surfactant (BLES), to which (2% by weight) chain-perdeuterated dipalmitoylphosphatidycholine (DPPG mixtures-d(62)) had been added, were studied using deuterium-NMR spectroscopy ((2)H-NMR) and differential scanning calorimetry (DSC). DSC scans of BLES showed a broad gel to liquid-crystalline phase transition between 10-35 degrees C, with a temperature of maximum heat flow (T(max)) around 27 degrees C. Incorporation of the DPPC-d(62) into BLES-reconstituted vesicles did not alter the T(max) or the transition range as observed by DSC or the hydrocarbon stretching modes of the lipids observed using infrared spectroscopy. Transition enthalpy change and (2)H-NMR order parameter profiles were not significantly altered by addition of calcium and cholesterol to BLES. (2)H-NMR spectra of the DPPC-d(62) probes in these samples were characteristic of a single average lipid environment at all temperatures. This suggested either continuous ordering of the bilayer through the transition during cooling or averaging of the DPPC-d(62) environment by rapid diffusion between small domains on a short timescale relative to that characteristic of the (2)H-NMR experiment. Addition of 10% by weight of soluble bovine serum albumin (1:0.1, BLES/albumin, dry wt/wt) broadened the transition slightly and resulted in the superposition of (2)H-NMR spectral features characteristic of coexisting fluid and ordered phases. This suggests the persistence of phase-separated domains throughout the transition regime (5-35 degrees C) of BLES with albumin. The study suggests albumin can cause segregation of protein bound-lipid domains in surfactant at NMR timescales (10(-5) s). Persistent phase separation at physiological temperature may provide for a basis for loss of surface activity of surfactant in dysfunction and disease.
Figures

Similar articles
-
An X-ray diffraction study of alterations in bovine lung surfactant bilayer structures induced by albumin.Chem Phys Lipids. 2006 Nov-Dec;144(2):137-45. doi: 10.1016/j.chemphyslip.2006.08.006. Epub 2006 Sep 8. Chem Phys Lipids. 2006. PMID: 17055468
-
Physicochemical studies on the interaction of serum albumin with pulmonary surfactant extract in films and bulk bilayer phase.J Colloid Interface Sci. 2010 Dec 15;352(2):456-64. doi: 10.1016/j.jcis.2010.08.058. Epub 2010 Sep 17. J Colloid Interface Sci. 2010. PMID: 20850129
-
Interfacial organizations of gel phospholipid and cholesterol in bovine lung surfactant films.Langmuir. 2007 Apr 10;23(8):4421-31. doi: 10.1021/la062513a. Epub 2007 Mar 7. Langmuir. 2007. PMID: 17341098
-
Structural hallmarks of lung surfactant: Lipid-protein interactions, membrane structure and future challenges.Arch Biochem Biophys. 2021 May 30;703:108850. doi: 10.1016/j.abb.2021.108850. Epub 2021 Mar 20. Arch Biochem Biophys. 2021. PMID: 33753033 Review.
-
[Organization of biomembranes as studied by 2H NMR (author's transl)].Seikagaku. 1980;52(3):172-7. Seikagaku. 1980. PMID: 6997410 Review. Japanese. No abstract available.
Cited by
-
Effects of palmitoylation on dynamics and phospholipid-bilayer-perturbing properties of the N-terminal segment of pulmonary surfactant protein SP-C as shown by 2H-NMR.Biophys J. 2008 Sep;95(5):2308-17. doi: 10.1529/biophysj.108.132845. Epub 2008 May 23. Biophys J. 2008. PMID: 18502795 Free PMC article.
-
Developing a Solution for Nasal and Olfactory Transport of Nanomaterials.Toxicol Pathol. 2022 Apr;50(3):329-343. doi: 10.1177/01926233221089209. Epub 2022 Apr 13. Toxicol Pathol. 2022. PMID: 35416103 Free PMC article.
-
Effects of the lung surfactant protein B construct Mini-B on lipid bilayer order and topography.Eur Biophys J. 2012 Sep;41(9):755-67. doi: 10.1007/s00249-012-0850-4. Epub 2012 Aug 19. Eur Biophys J. 2012. PMID: 22903196 Free PMC article.
-
Atomic force microscopy studies of functional and dysfunctional pulmonary surfactant films, II: albumin-inhibited pulmonary surfactant films and the effect of SP-A.Biophys J. 2008 Sep 15;95(6):2779-91. doi: 10.1529/biophysj.108.130732. Epub 2008 Jun 6. Biophys J. 2008. PMID: 18539636 Free PMC article.
References
-
- Goerke, J. 1998. Pulmonary surfactant: function and molecular composition. Biochim. Biophys. Acta. 1408:79–89. - PubMed
-
- Batenburg, J. J., and H. P. Haagsman. 1998. The lipids of pulmonary surfactant: dynamics and interactions with proteins. Prog. Lipid. Res. 37:235–276. - PubMed
-
- Enhorning, G. 1996. Pulmonary surfactant function in alveoli and conducting air-ways. Can. Respir. J. 3:21–27.
-
- Gaver III, D. P., O. E. Jensen, and D. Halpern. 2005. Surfactant and air-way liquid flows. In Lung Surfactant Function and Disorder. K. Nag, editor. CRC Press, Boca Raton, FL. 191–227.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

