Checkpoint functions are required for normal S-phase progression in Saccharomyces cerevisiae RCAF- and CAF-I-defective mutants
- PMID: 16501045
- PMCID: PMC1533778
- DOI: 10.1073/pnas.0511102103
Checkpoint functions are required for normal S-phase progression in Saccharomyces cerevisiae RCAF- and CAF-I-defective mutants
Abstract
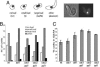
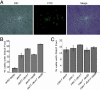
The chromatin-assembly factor I (CAF-I) and the replication-coupling assembly factor (RCAF) complexes function in chromatin assembly during DNA replication and repair and play a role in the maintenance of genome stability. Here, we have investigated their role in checkpoints and S-phase progression. FACS analysis of mutants lacking Asf1 or Cac1 as well as various checkpoint proteins indicated that normal rates of S-phase progression in asf1 mutants have a strong requirement for replication checkpoint proteins, whereas normal S-phase progression in cac1 mutants has only a weak requirement for either replication or DNA-damage checkpoint proteins. Furthermore, asf1 mutants had high levels of Ddc2.GFP foci that were further increased in asf1 dun1 double mutants consistent with a requirement for checkpoint proteins in S-phase progression in asf1 mutants, whereas cac1 mutants had much lower levels of Ddc2.GFP foci that were not increased by a dun1 mutation. Our data suggest that RCAF defects lead to unstable replication forks that are then stabilized by replication checkpoint proteins, whereas CAF-I defects likely cause different types of DNA damage.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures



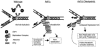
Similar articles
-
The Saccharomyces cerevisiae Rad6 postreplication repair and Siz1/Srs2 homologous recombination-inhibiting pathways process DNA damage that arises in asf1 mutants.Mol Cell Biol. 2009 Oct;29(19):5226-37. doi: 10.1128/MCB.00894-09. Epub 2009 Jul 27. Mol Cell Biol. 2009. PMID: 19635810 Free PMC article.
-
Chromatin assembly factors Asf1 and CAF-1 have overlapping roles in deactivating the DNA damage checkpoint when DNA repair is complete.Proc Natl Acad Sci U S A. 2009 Jan 27;106(4):1151-6. doi: 10.1073/pnas.0812578106. Epub 2009 Jan 21. Proc Natl Acad Sci U S A. 2009. PMID: 19164567 Free PMC article.
-
Regulation of histone deposition proteins Asf1/Hir1 by multiple DNA damage checkpoint kinases in Saccharomyces cerevisiae.Genetics. 2005 Nov;171(3):885-99. doi: 10.1534/genetics.105.044719. Epub 2005 Jul 14. Genetics. 2005. PMID: 16020781 Free PMC article.
-
The S-phase checkpoint: targeting the replication fork.Biol Cell. 2009 Aug 19;101(11):617-27. doi: 10.1042/BC20090053. Biol Cell. 2009. PMID: 19686094 Review.
-
Arrested replication fork processing: interplay between checkpoints and recombination.DNA Repair (Amst). 2007 Jul 1;6(7):1042-61. doi: 10.1016/j.dnarep.2007.02.024. Epub 2007 Apr 6. DNA Repair (Amst). 2007. PMID: 17412649 Review.
Cited by
-
Replication stress checkpoint signaling controls tRNA gene transcription.Nat Struct Mol Biol. 2010 Aug;17(8):976-81. doi: 10.1038/nsmb.1857. Epub 2010 Jul 18. Nat Struct Mol Biol. 2010. PMID: 20639887
-
Mutual interdependence of MSI1 (CAC3) and YAK1 in Saccharomyces cerevisiae.J Mol Biol. 2007 Apr 20;368(1):30-43. doi: 10.1016/j.jmb.2007.01.046. Epub 2007 Jan 23. J Mol Biol. 2007. PMID: 17321547 Free PMC article.
-
Regulation of Replication Fork Advance and Stability by Nucleosome Assembly.Genes (Basel). 2017 Jan 24;8(2):49. doi: 10.3390/genes8020049. Genes (Basel). 2017. PMID: 28125036 Free PMC article. Review.
-
Rapid analysis of Saccharomyces cerevisiae genome rearrangements by multiplex ligation-dependent probe amplification.PLoS Genet. 2012;8(3):e1002539. doi: 10.1371/journal.pgen.1002539. Epub 2012 Mar 1. PLoS Genet. 2012. PMID: 22396658 Free PMC article.
-
Histone H3K56 acetylation, CAF1, and Rtt106 coordinate nucleosome assembly and stability of advancing replication forks.PLoS Genet. 2011 Nov;7(11):e1002376. doi: 10.1371/journal.pgen.1002376. Epub 2011 Nov 10. PLoS Genet. 2011. PMID: 22102830 Free PMC article.
References
-
- Ye X., Franco A. A., Santos H., Nelson D. M., Kaufman P. D., Adams P. D. Mol. Cell. 2003;11:341–351. - PubMed
-
- Loyola A., Almouzni G. Biochim. Biophys. Acta. 2004;1677:3–11. - PubMed
-
- Smith S., Stillman B. Cell. 1989;58:15–25. - PubMed
-
- Gaillard P. H., Martini E. M., Kaufman P. D., Stillman B., Moustacchi E., Almouzni G. Cell. 1996;86:887–896. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

