Intranasal vaccination with recombinant adeno-associated virus type 5 against human papillomavirus type 16 L1
- PMID: 16501072
- PMCID: PMC1395428
- DOI: 10.1128/JVI.80.6.2621-2630.2006
Intranasal vaccination with recombinant adeno-associated virus type 5 against human papillomavirus type 16 L1
Abstract
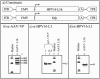
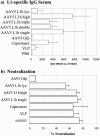
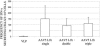
Adeno-associated viruses (AAV) have been developed and evaluated as recombinant vectors for gene therapy in many preclinical studies, as well as in clinical trials. However, only a few approaches have used recombinant AAV (rAAV) to deliver vaccine antigens. We generated an rAAV encoding the major capsid protein L1 (L1h) from the human papillomavirus type 16 (HPV16), aiming to develop a prophylactic vaccine against HPV16 infections, which are the major cause of cervical cancer in women worldwide. A single dose of rAAV5 L1h administered intranasally was sufficient to induce high titers of L1-specific serum antibodies, as well as mucosal antibodies in vaginal washes. Seroconversion was maintained for at least 1 year. In addition, a cellular immune response was still detectable 60 weeks after immunization. Furthermore, lyophilized rAAV5 L1h successfully evoked a systemic and mucosal immune response in mice. These data clearly show the efficacy of a single-dose intranasal immunization against HPV16 based on the recombinant rAAV5L1h vector without the need of an adjuvant.
Figures



References
-
- Balmelli, C., S. Demotz, H. Acha-Orbea, P. De Grandi, and D. Nardelli-Haefliger. 2002. Trachea, lung, and tracheobronchial lymph nodes are the major sites where antigen-presenting cells are detected after nasal vaccination of mice with human papillomavirus type 16 virus-like particles. J. Virol. 76:12596-12602. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

