Inhibition of interferon signaling by rabies virus phosphoprotein P: activation-dependent binding of STAT1 and STAT2
- PMID: 16501077
- PMCID: PMC1395475
- DOI: 10.1128/JVI.80.6.2675-2683.2006
Inhibition of interferon signaling by rabies virus phosphoprotein P: activation-dependent binding of STAT1 and STAT2
Abstract
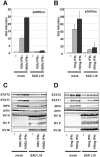
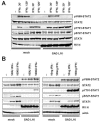
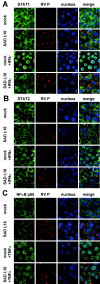
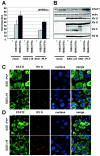
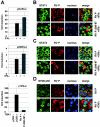
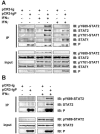
Rabies virus (RV) phosphoprotein P is an interferon (IFN) antagonist counteracting transcriptional activation of type I IFN (K. Brzózka, S. Finke, and K. K. Conzelmann, J. Virol 79:7673-7681, 2005). We here show that RV P in addition is responsible for preventing IFN-alpha/beta- and IFN-gamma-stimulated JAK-STAT signaling in RV-infected cells by the retention of activated STATs in the cytoplasm. Expression of IFN-stimulated response element- and gamma-activated sequence-controlled genes was severely impaired in cells infected with RV SAD L16 or in cells expressing RV P protein from transfected plasmids. In contrast, a recombinant RV expressing small amounts of P had lost the ability to interfere with JAK-STAT signaling. IFN-mediated tyrosine phosphorylation of STAT1 and STAT2 was not impaired in RV P-expressing cells; rather, a defect in STAT recycling was suggested by distinct accumulation of tyrosine-phosphorylated STATs in cell extracts. In the presence of P, activated STAT1 and STAT2 were unable to accumulate in the nucleus. Notably, STAT1 and STAT2 were coprecipitated with RV P only from extracts of cells previously stimulated with IFN-alpha or IFN-gamma, whereas in nonstimulated cells no association of P with STATs was observed. This conditional, IFN activation-dependent binding of tyrosine-phosphorylated STATs by RV P is unique for a viral IFN antagonist. The 10 C-terminal residues of P are required for counteracting JAK-STAT signaling but not for inhibition of transcriptional activation of IFN-beta, thus demonstrating two independent functions of RV P in counteracting the host's IFN response.
Figures
References
-
- Aaronson, D. S., and C. M. Horvath. 2002. A road map for those who don't know JAK-STAT. Science 296:1653-1655. - PubMed
-
- Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. - PubMed
-
- Bowie, A. G., and I. R. Haga. 2005. The role of Toll-like receptors in the host response to viruses. Mol. Immunol. 42:859-867. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

