Annexin 2: a novel human immunodeficiency virus type 1 Gag binding protein involved in replication in monocyte-derived macrophages
- PMID: 16501079
- PMCID: PMC1395445
- DOI: 10.1128/JVI.80.6.2694-2704.2006
Annexin 2: a novel human immunodeficiency virus type 1 Gag binding protein involved in replication in monocyte-derived macrophages
Abstract
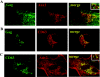
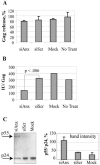
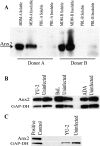
Human immunodeficiency virus (HIV) replication in the major natural target cells, CD4+ T lymphocytes and macrophages, is parallel in many aspects of the virus life cycle. However, it differs as to viral assembly and budding, which take place on plasma membranes in T cells and on endosomal membranes in macrophages. It has been postulated that cell type-specific host factors may aid in directing viral assembly to distinct destinations. In this study we defined annexin 2 (Anx2) as a novel HIV Gag binding partner in macrophages. Anx2-Gag binding was confined to productively infected macrophages and was not detected in quiescently infected monocyte-derived macrophages (MDM) in which an HIV replication block was mapped to the late stages of the viral life cycle (A. V. Albright, R. M. Vos, and F. Gonzalez-Scarano, Virology 325:328-339, 2004). We demonstrate that the Anx2-Gag interaction likely occurs at the limiting membranes of late endosomes/multivesicular bodies and that Anx2 depletion is associated with a significant decline in the infectivity of released virions; this coincided with incomplete Gag processing and inefficient incorporation of CD63. Cumulatively, our data suggest that Anx2 is essential for the proper assembly of HIV in MDM.
Figures




References
-
- Abdurahman, S., S. Hoglund, L. Goobar-Larsson, and A. Vahlne. 2004. Selected amino acid substitutions in the C-terminal region of human immunodeficiency virus type 1 capsid protein affect virus assembly and release. J. Gen. Virol. 85:2903-2913. - PubMed
-
- Adamson, C. S., A. Davies, Y. Soneoka, M. Nermut, K. Mitrophanous, and I. M. Jones. 2003. A block in virus-like particle maturation following assembly of murine leukaemia virus in insect cells. Virology 314:488-496. - PubMed
-
- Albright, A. V., J. T. Shieh, M. J. O'Connor, and Gonzalez-Scarano, F. 2000. Characterization of cultured microglia that can be infected by HIV-1. J. Neurovirol. 6(Suppl. 1):S53-S60. - PubMed
-
- Albright, A. V., R. M. Vos, and F. Gonzalez-Scarano. 2004. Low-level HIV replication in mixed glial cultures is associated with alterations in the processing of p55(Gag). Virology 325:328-339. - PubMed
-
- Brownstein, C., A. B. Deora, A. T. Jacovina, R. Weintraub, M. Gertler, K. M. Khan, D. J. Falcone, and K. A. Hajjar. 2004. Annexin II mediates plasminogen-dependent matrix invasion by human monocytes: enhanced expression by macrophages. Blood 103:317-324. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

