Improved gene delivery to intestinal mucosa by adenoviral vectors bearing subgroup B and d fibers
- PMID: 16501084
- PMCID: PMC1395461
- DOI: 10.1128/JVI.80.6.2747-2759.2006
Improved gene delivery to intestinal mucosa by adenoviral vectors bearing subgroup B and d fibers
Abstract
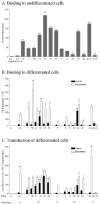
A major obstacle to successful oral vaccination is the lack of antigen delivery systems that are both safe and highly efficient. Conventional replication-incompetent adenoviral vectors, derived from human adenoviruses of subgroup C, are poorly efficient in delivering genetic material to differentiated intestinal epithelia. To date, 51 human adenovirus serotypes have been identified and shown to recognize different cellular receptors with different tissue distributions. This natural diversity was exploited in the present study to identify suitable adenoviral vectors for efficient gene delivery to the human intestinal epithelium. In particular, we compared the capacities of a library of adenovirus type 5-based vectors pseudotyped with fibers of several human serotypes for transduction, binding, and translocation toward the basolateral pole in human and murine tissue culture models of differentiated intestinal epithelia. In addition, antibody-based inhibition was used to gain insight into the molecular interactions needed for efficient attachment. We found that vectors differing merely in their fiber proteins displayed vastly different capacities for gene transfer to differentiated human intestinal epithelium. Notably, vectors bearing fibers derived from subgroup B and subgroup D serotypes transduced the apical pole of human epithelium with considerably greater efficiency than a subgroup C vector. Such efficiency was correlated with the capacity to use CD46 or sialic acid-containing glycoconjugates as opposed to CAR as attachment receptors. These results suggest that substantial gains could be made in gene transfer to digestive epithelium by exploiting the tropism of existing serotypes of human adenoviruses.
Figures
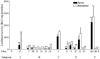
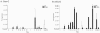
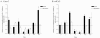

Similar articles
-
Endowing human adenovirus serotype 5 vectors with fiber domains of species B greatly enhances gene transfer into human mesenchymal stem cells.Stem Cells. 2005 Nov-Dec;23(10):1598-607. doi: 10.1634/stemcells.2005-0016. Stem Cells. 2005. PMID: 16293583
-
Optimization of adenovirus serotype 35 vectors for efficient transduction in human hematopoietic progenitors: comparison of promoter activities.Gene Ther. 2005 Oct;12(19):1424-33. doi: 10.1038/sj.gt.3302562. Gene Ther. 2005. PMID: 15944730
-
A 50-kDa membrane protein mediates sialic acid-independent binding and infection of conjunctival cells by adenovirus type 37.Virology. 2001 Jan 5;279(1):78-89. doi: 10.1006/viro.2000.0703. Virology. 2001. PMID: 11145891
-
Adenovirus vectors composed of subgroup B adenoviruses.Curr Gene Ther. 2007 Aug;7(4):229-38. doi: 10.2174/156652307781369137. Curr Gene Ther. 2007. PMID: 17969556 Review.
-
Intracellular trafficking of adenovirus: many means to many ends.Adv Drug Deliv Rev. 2007 Aug 10;59(8):810-21. doi: 10.1016/j.addr.2007.06.007. Epub 2007 Jun 28. Adv Drug Deliv Rev. 2007. PMID: 17707546 Review.
Cited by
-
Firewalls Prevent Systemic Dissemination of Vectors Derived from Human Adenovirus Type 5 and Suppress Production of Transgene-Encoded Antigen in a Murine Model of Oral Vaccination.Front Cell Infect Microbiol. 2018 Jan 25;8:6. doi: 10.3389/fcimb.2018.00006. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29423380 Free PMC article.
-
Human species D adenovirus hexon capsid protein mediates cell entry through a direct interaction with CD46.Proc Natl Acad Sci U S A. 2021 Jan 19;118(3):e2020732118. doi: 10.1073/pnas.2020732118. Proc Natl Acad Sci U S A. 2021. PMID: 33384338 Free PMC article.
-
Overview of the Trending Enteric Viruses and Their Pathogenesis in Intestinal Epithelial Cell Infection.Biomedicines. 2024 Dec 5;12(12):2773. doi: 10.3390/biomedicines12122773. Biomedicines. 2024. PMID: 39767680 Free PMC article. Review.
-
Influence of coagulation factor zymogens on the infectivity of adenoviruses pseudotyped with fibers from subgroup D.J Virol. 2007 Apr;81(7):3627-31. doi: 10.1128/JVI.02786-06. Epub 2007 Jan 24. J Virol. 2007. PMID: 17251290 Free PMC article.
-
Targeting the gastrointestinal tract with viral vectors: state of the art and possible applications in research and therapy.Histochem Cell Biol. 2016 Dec;146(6):709-720. doi: 10.1007/s00418-016-1496-6. Epub 2016 Sep 24. Histochem Cell Biol. 2016. PMID: 27665281 Review.
References
-
- Ambriovic-Ristov, A., S. Mercier, and M. Eloit. 2003. Shortening adenovirus type 5 fiber shaft decreases the efficiency of postbinding steps in CAR-expressing and nonexpressing cells. Virology 312:425-433. - PubMed
-
- Arcasoy, S. M., J. Latoche, M. Gondor, S. C. Watkins, R. A. Henderson, R. Hughey, O. J. Finn, and J. M. Pilewski. 1997. MUC1 and other sialoglycoconjugates inhibit adenovirus-mediated gene transfer to epithelial cells. Am. J. Respir. Cell Mol. Biol. 17:422-435. - PubMed
-
- Balzar, M., F. A. Prins, H. A. Bakker, G. J. Fleuren, S. O. Warnaar, and S. V. Litvinov. 1999. The structural analysis of adhesions mediated by Ep-CAM. Exp. Cell Res. 246:108-121. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

