Beta1 integrin mediates internalization of mammalian reovirus
- PMID: 16501085
- PMCID: PMC1395463
- DOI: 10.1128/JVI.80.6.2760-2770.2006
Beta1 integrin mediates internalization of mammalian reovirus
Abstract
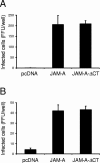
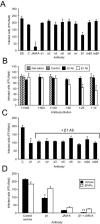
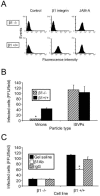
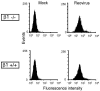
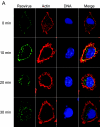
Reovirus infection is initiated by interactions between the attachment protein sigma1 and cell surface carbohydrate and junctional adhesion molecule A (JAM-A). Expression of a JAM-A mutant lacking a cytoplasmic tail in nonpermissive cells conferred full susceptibility to reovirus infection, suggesting that cell surface molecules other than JAM-A mediate viral internalization following attachment. The presence of integrin-binding sequences in reovirus outer capsid protein lambda2, which serves as the structural base for sigma1, suggests that integrins mediate reovirus endocytosis. A beta1 integrin-specific antibody, but not antibodies specific for other integrin subunits, inhibited reovirus infection of HeLa cells. Expression of a beta1 integrin cDNA, along with a cDNA encoding JAM-A, in nonpermissive chicken embryo fibroblasts conferred susceptibility to reovirus infection. Infectivity of reovirus was significantly reduced in beta1-deficient mouse embryonic stem cells in comparison to isogenic cells expressing beta1. However, reovirus bound equivalently to cells that differed in levels of beta1 expression, suggesting that beta1 integrins are involved in a postattachment entry step. Concordantly, uptake of reovirus virions into beta1-deficient cells was substantially diminished in comparison to viral uptake into beta1-expressing cells. These data provide evidence that beta1 integrin facilitates reovirus internalization and suggest that viral entry occurs by interactions of reovirus virions with independent attachment and entry receptors on the cell surface.
Figures





Similar articles
-
JAM-A-independent, antibody-mediated uptake of reovirus into cells leads to apoptosis.J Virol. 2006 Feb;80(3):1261-70. doi: 10.1128/JVI.80.3.1261-1270.2006. J Virol. 2006. PMID: 16415003 Free PMC article.
-
Junctional adhesion molecule a serves as a receptor for prototype and field-isolate strains of mammalian reovirus.J Virol. 2005 Jul;79(13):7967-78. doi: 10.1128/JVI.79.13.7967-7978.2005. J Virol. 2005. PMID: 15956543 Free PMC article.
-
Reovirus directly engages integrin to recruit clathrin for entry into host cells.Nat Commun. 2021 Apr 12;12(1):2149. doi: 10.1038/s41467-021-22380-0. Nat Commun. 2021. PMID: 33846319 Free PMC article.
-
Attachment and cell entry of mammalian orthoreovirus.Curr Top Microbiol Immunol. 2006;309:1-38. doi: 10.1007/3-540-30773-7_1. Curr Top Microbiol Immunol. 2006. PMID: 16909895 Review.
-
From touchdown to transcription: the reovirus cell entry pathway.Curr Top Microbiol Immunol. 2010;343:91-119. doi: 10.1007/82_2010_32. Curr Top Microbiol Immunol. 2010. PMID: 20397070 Free PMC article. Review.
Cited by
-
Structure-Activity Relationships of RGD-Containing Peptides in Integrin αvβ5-Mediated Cell Adhesion.ACS Omega. 2023 Jan 24;8(5):4687-4693. doi: 10.1021/acsomega.2c06540. eCollection 2023 Feb 7. ACS Omega. 2023. PMID: 36777587 Free PMC article.
-
NPXY motifs in the beta1 integrin cytoplasmic tail are required for functional reovirus entry.J Virol. 2008 Apr;82(7):3181-91. doi: 10.1128/JVI.01612-07. Epub 2008 Jan 23. J Virol. 2008. PMID: 18216114 Free PMC article.
-
Integrin αvβ5 Internalizes Zika Virus during Neural Stem Cells Infection and Provides a Promising Target for Antiviral Therapy.Cell Rep. 2020 Jan 28;30(4):969-983.e4. doi: 10.1016/j.celrep.2019.11.020. Epub 2020 Jan 16. Cell Rep. 2020. PMID: 31956073 Free PMC article.
-
Lipids Cooperate with the Reovirus Membrane Penetration Peptide to Facilitate Particle Uncoating.J Biol Chem. 2016 Dec 23;291(52):26773-26785. doi: 10.1074/jbc.M116.747477. Epub 2016 Nov 15. J Biol Chem. 2016. PMID: 27875299 Free PMC article.
-
Baculovirus-assisted Reovirus Infection in Monolayer and Spheroid Cultures of Glioma cells.Sci Rep. 2017 Dec 15;7(1):17654. doi: 10.1038/s41598-017-17709-z. Sci Rep. 2017. PMID: 29247249 Free PMC article.
References
-
- Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2002. Integrin α3b1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108:407-419. - PubMed
-
- Barton, E. S., J. L. Connolly, J. C. Forrest, J. D. Chappell, and T. S. Dermody. 2001. Utilization of sialic acid as a coreceptor enhances reovirus attachment by multistep adhesion strengthening. J. Biol. Chem. 276:2200-2211. - PubMed
-
- Barton, E. S., J. C. Forrest, J. L. Connolly, J. D. Chappell, Y. Liu, F. Schnell, A. Nusrat, C. A. Parkos, and T. S. Dermody. 2001. Junction adhesion molecule is a receptor for reovirus. Cell 104:441-451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

