Detection of cell-cell fusion mediated by Ebola virus glycoproteins
- PMID: 16501090
- PMCID: PMC1395460
- DOI: 10.1128/JVI.80.6.2815-2822.2006
Detection of cell-cell fusion mediated by Ebola virus glycoproteins
Abstract
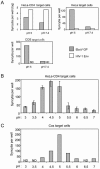
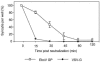
Ebola viruses (EboV) are enveloped RNA viruses infecting cells by a pH-dependent process mediated by viral glycoproteins (GP) involving endocytosis of virions and their routing into acidic endosomes. As with well-characterized pH-dependent viral entry proteins, in particular influenza virus hemagglutinin, it is thought that EboV GP require activation by low pH in order to mediate fusion of the viral envelope with the membrane of endosomes. However, it has not yet been possible to confirm the direct role of EboV GP in membrane fusion and the requirement for low-pH activation. It was in particular not possible to induce formation of syncytia by exposing cells expressing EboV GP to acidic medium. Here, we have used an assay based on the induction of a beta-galactosidase (lacZ) reporter gene in target cells to detect cytoplasmic exchanges, indicating membrane fusion, with cells expressing EboV GP (Zaire species). Acidic activation of GP-expressing cells was required for efficient fusion with target cells. The direct role of EboV GP in this process is indicated by its inhibition by anti-GP antibodies and by the lack of activity of mutant GP normally expressed at the cell surface but defective for virus entry. Fusion was not observed when target cells underwent acidic treatment, for example, when they were placed in coculture with GP-expressing cells before the activation step. This unexpected feature, possibly related to the nature of the EboV receptor, could explain the impossibility of inducing formation of syncytia among GP-expressing cells.
Figures



References
-
- Chan, S. Y., C. J. Empig, F. J. Welte, R. F. Speck, A. Schmaljohn, J. F. Kreisberg, and M. A. Goldsmith. 2001. Folate receptor-alpha is a cofactor for cellular entry by Marburg and Ebola viruses. Cell 106:117-126. - PubMed
-
- Chan, S. Y., M. C. Ma, and M. A. Goldsmith. 2000. Differential induction of cellular detachment by envelope glycoproteins of Marburg and Ebola (Zaire) viruses. J. Gen. Virol. 81:2155-2159. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

