Innate immune responses to herpes simplex virus type 2 influence skin homing molecule expression by memory CD4+ lymphocytes
- PMID: 16501095
- PMCID: PMC1395438
- DOI: 10.1128/JVI.80.6.2863-2872.2006
Innate immune responses to herpes simplex virus type 2 influence skin homing molecule expression by memory CD4+ lymphocytes
Abstract
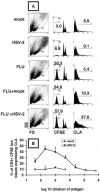
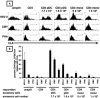
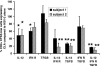
Herpes simplex virus (HSV) infections of humans are characterized by intermittent, lytic replication in epithelia. Circulating HSV-specific CD4 T cells express lower levels of preformed cutaneous lymphocyte-associated antigen (CLA), a skin-homing receptor, than do circulating HSV-specific CD8 T cells but, paradoxically, move into infected skin earlier than CD8 cells. Memory CD4 T cells develop strong and selective expression of CLA and E-selectin ligand while responding to HSV antigen in vitro. We now show that interleukin-12, type I interferon, and transforming growth factor beta are each involved in CLA expression by memory HSV type 2 (HSV-2)-specific CD4 T cells in peripheral blood mononuclear cells (PBMC). A reduction of the number of monocytes and dendritic cells from PBMC reduces CLA expression by HSV-2-responsive CD4 lymphoblasts, while their reintroduction restores this phenotype, identifying these cells as possible sources of CLA-promoting cytokines. Plasmacytoid dendritic cells are particularly potent inducers of CLA on HSV-reactive CD4 T cells. These observations are consistent with cooperation between innate and acquired immunity to promote a pattern of homing receptor expression that is physiologically appropriate for trafficking to infected tissues.
Figures


References
-
- Allan, R. S., C. M. Smith, G. T. Belz, A. L. van Lint, L. M. Wakim, W. R. Heath, and F. R. Carbone. 2003. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science 301:1925-1928. - PubMed
-
- Ankel, H., D. F. Westra, S. Welling-Wester, and P. Lebon. 1998. Induction of interferon-alpha by glycoprotein D of herpes simplex virus: a possible role of chemokine receptors. Virology 251:317-326. - PubMed
-
- Armerding, D., and T. S. Kupper. 1999. Functional cutaneous lymphocyte antigen can be induced in essentially all peripheral blood T lymphocytes. Int. Arch. Allergy Immunol. 119:212-222. - PubMed
-
- Bangert, C., J. Friedl, G. Stary, G. Stingl, and T. Kopp. 2003. Immunopathologic features of allergic contact dermatitis in humans: participation of plasmacytoid dendritic cells in the pathogenesis of the disease? J. Investig. Dermatol. 121:1409-1418. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

