A small loop in the capsid protein of Moloney murine leukemia virus controls assembly of spherical cores
- PMID: 16501097
- PMCID: PMC1395457
- DOI: 10.1128/JVI.80.6.2884-2893.2006
A small loop in the capsid protein of Moloney murine leukemia virus controls assembly of spherical cores
Abstract
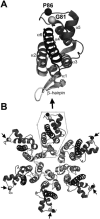
We report the identification of a novel domain in the Gag protein of Moloney murine leukemia virus (MoLV) that is important for the formation of spherical cores. Analysis of 18 insertional mutations in the N-terminal domain of the capsid protein (CA) identified 3 that were severely defective for viral assembly and release. Transmission electron microscopy of cells producing these mutants showed assembly of Gag proteins in large, flat or dome-shaped patches at the plasma membrane. Spherical cores were not formed, and viral particles were not released. This late assembly/release block was partially rescued by wild-type virus. All three mutations localized to the small loop between alpha-helices 4 and 5 of CA, analogous to the cyclophilin A-binding loop of human immunodeficiency virus type 1 CA. In the X-ray structure of the hexameric form of MLV CA, this loop is located at the periphery of the hexamer. The phenotypes of mutations in this loop suggest that formation of a planar lattice of Gag is unhindered by mutations in the loop. However, the lack of progression of these planar structures to spherical ones suggests that mutations in this loop may prevent formation of pentamers or of stable pentamer-hexamer interactions, which are essential for the formation of a closed, spherical core. This region in CA, focused to a few residues of a small loop, may offer a novel therapeutic target for retroviral diseases.
Figures


References
-
- Bouamr, F., J. A. Melillo, M. Q. Wang, K. Nagashima, M. de Los Santos, A. Rein, and S. P. Goff. 2003. PPPYVEPTAP motif is the late domain of human T-cell leukemia virus type 1 Gag and mediates its functional interaction with cellular proteins Nedd4 and Tsg101 [corrected]. J. Virol. 77:11882-11895. - PMC - PubMed
-
- Briggs, J. A., M. N. Simon, I. Gross, H. G. Krausslich, S. D. Fuller, V. M. Vogt, and M. C. Johnson. 2004. The stoichiometry of Gag protein in HIV-1. Nat. Struct. Mol. Biol. 11:672-675. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

