Recruitment of the adaptor protein 2 complex by the human immunodeficiency virus type 2 envelope protein is necessary for high levels of virus release
- PMID: 16501101
- PMCID: PMC1395462
- DOI: 10.1128/JVI.80.6.2924-2932.2006
Recruitment of the adaptor protein 2 complex by the human immunodeficiency virus type 2 envelope protein is necessary for high levels of virus release
Abstract
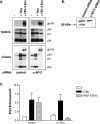
The envelope (Env) protein of human immunodeficiency virus type 2 (HIV-2) and the HIV-1 Vpu protein stimulate the release of retroviral particles from human cells that restrict virus production, an activity that we call the enhancement of virus release (EVR). We have previously shown that two separate domains in the HIV-2 envelope protein are required for this activity: a glycine-tyrosine-x-x-hydrophobic (GYxxtheta) motif in the cytoplasmic tail and an unmapped region in the ectodomain of the protein. We here report that the cellular partner of the GYxxtheta motif is the adaptor protein complex AP-2. The mutation of this motif or the depletion of AP-2 by RNA interference abrogated EVR activity and changed the cellular distribution of the Env from a predominantly punctate pattern to a more diffuse distribution. Since the L domain of equine infectious anemia virus (EIAV) contains a Yxxtheta motif that interacts with AP-2, we used both wild-type and L domain-defective particles of HIV-1 and EIAV to examine whether the HIV-2 Env EVR function was analogous to L domain activity. We observed that the production of all particles was stimulated by HIV-2 Env or Vpu, suggesting that the L domain and EVR activities play independent roles in the release of retroviruses. Interestingly, we found that the cytoplasmic tail of the murine leukemia virus (MLV) Env could functionally substitute for the HIV-2 Env tail, but it did so in a manner that did not require a Yxxtheta motif or AP-2. The cellular distribution of the chimeric HIV-2/MLV Env was significantly less punctate than the wild-type Env, although confocal analysis revealed an overlap in the steady-state locations of the two proteins. Taken together, these data suggest that the essential GYxxtheta motif in the HIV-2 Env tail recruits AP-2 in order to direct Env to a cellular pathway or location that is necessary for its ability to enhance virus release but that an alternate mechanism provided by the MLV Env tail can functionally substitute.
Figures





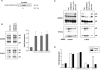
References
-
- Balliet, J. W., D. L. Kolson, G. Eiger, F. M. Kim, K. A. McGann, A. Srinivasan, and R. Collman. 1994. Distinct effects in primary macrophages and lymphocytes of the human immunodeficiency virus type 1 accessory genes vpr, vpu, and nef: mutational analysis of a primary HIV-1 isolate. Virology 200:623-631. - PubMed
-
- Batonick, M., M. Favre, M. Boge, P. Spearman, S. Honing, and M. Thali. 2005. Interaction of HIV-1 Gag with the clathrin-associated adaptor AP-2. Virology 342:190-200. - PubMed
-
- Boge, M., S. Wyss, J. S. Bonifacino, and M. Thali. 1998. A membrane-proximal tyrosine-based signal mediates internalization of the HIV-1 envelope glycoprotein via interaction with the AP-2 clathrin adaptor. J. Biol. Chem. 273:15773-15778. - PubMed
-
- Bonifacino, J. S., and L. M. Traub. 2003. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72:395-447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

