Infection of dendritic cells (DCs), not DC-SIGN-mediated internalization of human immunodeficiency virus, is required for long-term transfer of virus to T cells
- PMID: 16501104
- PMCID: PMC1395470
- DOI: 10.1128/JVI.80.6.2949-2957.2006
Infection of dendritic cells (DCs), not DC-SIGN-mediated internalization of human immunodeficiency virus, is required for long-term transfer of virus to T cells
Abstract
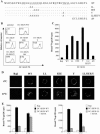
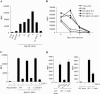
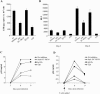
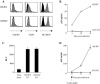
The C-type lectin DC-SIGN expressed on immature dendritic cells (DCs) captures human immunodeficiency virus (HIV) particles and enhances the infection of CD4+ T cells. This process, known as trans-enhancement of T-cell infection, has been related to HIV endocytosis. It has been proposed that DC-SIGN targets HIV to a nondegradative compartment within DCs and DC-SIGN-expressing cells, allowing incoming virus to persist for several days before infecting target cells. In this study, we provide several lines of evidence suggesting that intracellular storage of intact virions does not contribute to HIV transmission. We show that endocytosis-defective DC-SIGN molecules enhance T-cell infection as efficiently as their wild-type counterparts, indicating that DC-SIGN-mediated HIV internalization is dispensable for trans-enhancement. Furthermore, using immature DCs that are genetically resistant to infection, we demonstrate that several days after viral uptake, HIV transfer from DCs to T cells requires viral fusion and occurs exclusively through DC infection and transmission of newly synthesized viral particles. Importantly, our results suggest that DC-SIGN participates in this process by cooperating with the HIV entry receptors to facilitate cis-infection of immature DCs and subsequent viral transfer to T cells. We suggest that such a mechanism, rather than intracellular storage of incoming virus, accounts for the long-term transfer of HIV to CD4+ T cells and may contribute to the spread of infection by DCs.
Figures
References
-
- Amara, A., A. Vidy, G. Boulla, K. Mollier, J. Garcia-Perez, J. Alcami, C. Blanpain, M. Parmentier, J. L. Virelizier, P. Charneau, and F. Arenzana-Seisdedos. 2003. G protein-dependent CCR5 signaling is not required for efficient infection of primary T lymphocytes and macrophages by R5 human immunodeficiency virus type 1 isolates. J. Virol. 77:2550-2558. - PMC - PubMed
-
- Canque, B., Y. Bakri, S. Camus, M. Yagello, A. Benjouad, and J. C. Gluckman. 1999. The susceptibility to X4 and R5 human immunodeficiency virus-1 strains of dendritic cells derived in vitro from CD34(+) hematopoietic progenitor cells is primarily determined by their maturation stage. Blood 93:3866-3875. - PubMed
-
- Deng, H. K., D. Unutmaz, V. N. KewalRamani, and D. R. Littman. 1997. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature 388:296-300. - PubMed
-
- Engering, A., T. B. Geijtenbeek, S. J. van Vliet, M. Wijers, E. van Liempt, N. Demaurex, A. Lanzavecchia, J. Fransen, C. G. Figdor, V. Piguet, and Y. van Kooyk. 2002. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J. Immunol. 168:2118-2126. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

